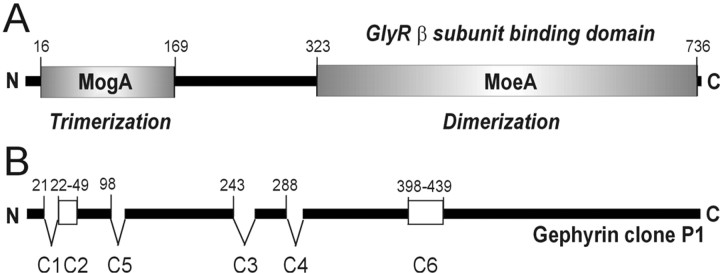
Figure 1.
Gephyrin structure. A, Gephyrin is composed of two domains that are homologous to the bacterial proteins MogA and MoeA and separated by a linker region. The N-terminal domain is involved in trimer formation, whereas the C-terminal domain mediates dimerization and is required for GlyR binding (Rees et al., 2003). Together, both domains are presumably involved in the assembly of an ordered hexagonal lattice. B, The initially identified clone P1 (Prior et al., 1992) bearing cassettes C2 and C6. Insertion of at least six distinct cassettes generates a large number of alternatively spliced gephyrin isoforms. Numbering identifies amino acid positions within the gephyrin open reading frame. N and C termini are indicated.