Figure 2.
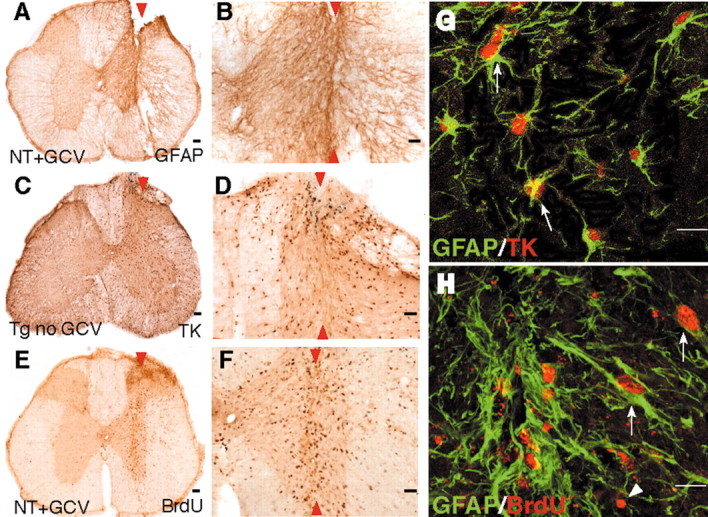
Expression of GFAP and transgene-derived TK by dividing reactive astrocytes adjacent to stab SCI in control mice. A-H, Transverse sections of upper lumbar spinal cord after longitudinal stab SCI (arrowheads) in either nontransgenic mice given GCV (A, B, E, F, H; NT+GCV) or GFAP-TK transgenic mice not given GCV (C, D, G; Tg no GCV). B, D, F, Details, respectively, of A, C, and E. A-F, Single immunohistochemical staining for GFAP (A, B), TK (C, D), or BrdU (E, F) viewed by bright-field microscopy. G, H, Double immunohistochemical staining for GFAP and TK (G) or GFAP and BrdU (H) viewed by laser-scanning confocal microscopy. Staining for GFAP is prominent in astrocyte processes and rare in cell bodies (A, B, G), whereas staining for TK is prominent in astrocyte cell bodies and rare in cell processes (C, D, G). After stab SCI, astrocytes along the wound margin hypertrophy and upregulate their expression of both GFAP (A, B, G) and TK (C, D, G). GCV had no effect on astrocyte scar formation in nontransgenic mice (A, B, E, F). Double staining and analysis using laser-scanning confocal microscopy reveal that TK staining is found only in GFAP-positive astrocytes (G, arrows). Many cells along the wound margin divide and take up BrdU (E, F). Many of these dividing BrdU-labeled cells are GFAP-positive reactive astrocytes (H, arrows); others are not (G, arrowheads). Scale bars: A, C, E, 100 μm; B, D, F, 50 μm; G, H, 15 μm.
