Figure 9.
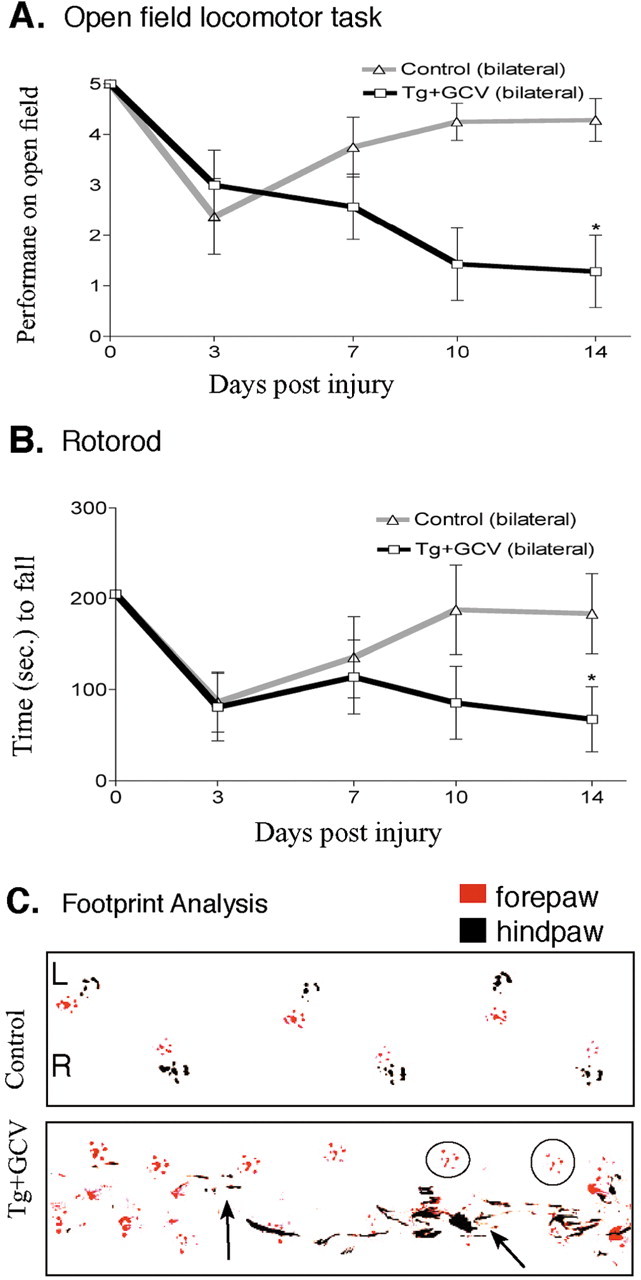
Persisting loss of motor function after ablation of reactive astrocytes in crush SCI. A, Time course of left hindlimb locomotor performance in an open field over 14 d after moderately severe forceps crush SCI in control mice and GFAP-TK transgenic mice given GCV (Tg+GCV). Control mice (n = 8) exhibited an initial impairment of bilateral (both sides scored individually and averaged) hindlimb performance after crush SCI that was fully reversible by 14 d. In contrast, transgenic mice (n = 7) given GCV failed to recover bilateral hindlimb performance during and after ablation of reactive astrocytes and by 14 d exhibited a substantial and significant impairment. *Significantly different from control, p < 0.05 (ANOVA plus post hoc pair-wise analysis). B, Time course of rotorod performance after SCI in control mice and GFAP-TK transgenic mice given GCV. Control mice (n = 8) exhibited an initial mild impairment of rotorod performance after crush SCI that was fully reversible by 14 d. In contrast, transgenic mice (n = 7) given GCV failed to recover rotorod performance during and after ablation of reactive astrocytes and by 14 d exhibited a substantial and significant impairment. *Significantly different from control, p < 0.05 (ANOVA plus post hoc pair-wise analysis). C, Representative footprint analysis at 14 d after crush SCI of a control mouse and a GFAP-TK transgenic mouse given GCV. Footprints of a control mouse illustrate normal plantar placement of forepaws and hindpaws. In contrast, footprints of a transgenic mouse given GCV exhibit smears on the left side (L, left arrow) indicative of toe dragging as well as frequent failure of plantar placement of the left hindpaw (circles show placement of only the left forepaw) indicative of dorsal foot dragging, and on the right (R) side, the hindpaw shows substantial smears (right arrow) indicative of toe dragging and uncoordinated hindpaw placements.
