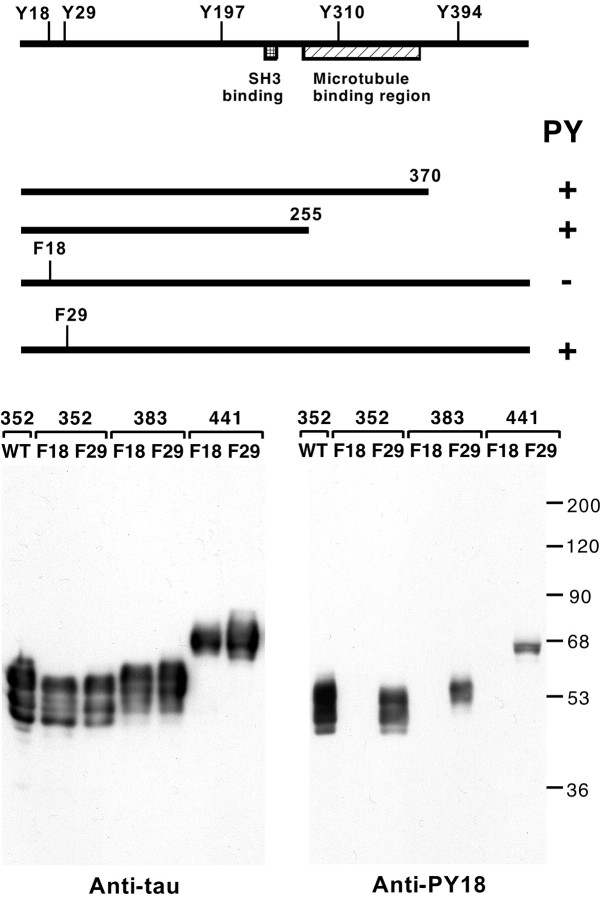
Figure 1.
Identification of tyr18 as a site for tyrosine phosphorylation in tau. A, The schematic drawing shows the deletion constructs and point mutants tested for tyrosine phosphorylation (PY) in the presence of fyn using cotransfected COS7 cells. All constructs tested were derived from the 352 residue human tau isoform, shown at the top with the positions of tyrosines marked (numbering is according to the 441 adult tau isoform; the 441 adult isoform does not contain any additional tyrosines). Two C terminal deletions and two point mutants were tested. After cotransfection with fyn into COS7 cells, tau was immunoprecipitated and tested for tyrosine phosphorylation with anti-phosphotyrosine. These data identified tyr18 as a tyrosine phosphorylation site in tau. B, An affinity-purified polyclonal antibody prepared against phosphorylated tyr18 (anti-PY18) was tested on COS7 cells cotransfected with fyn and various tau constructs. Lysates were probed with anti-PY18 (right panel) or a mixture of anti-tau monoclonal antibodies as control (left panel). A fyn control blot was also performed, showing that similar amounts of fyn were expressed in each transfection (data not shown). Mutants of three tau isoforms (amino acid lengths indicated) were tested. Mutations converted either tyr18 to phe18 (F18) or tyr29 to phe29 (F29). WT is wild-type tau. Despite the presence of tau in each transfection, F18 mutants did not react with anti-PY18, whereas F29 mutants did.