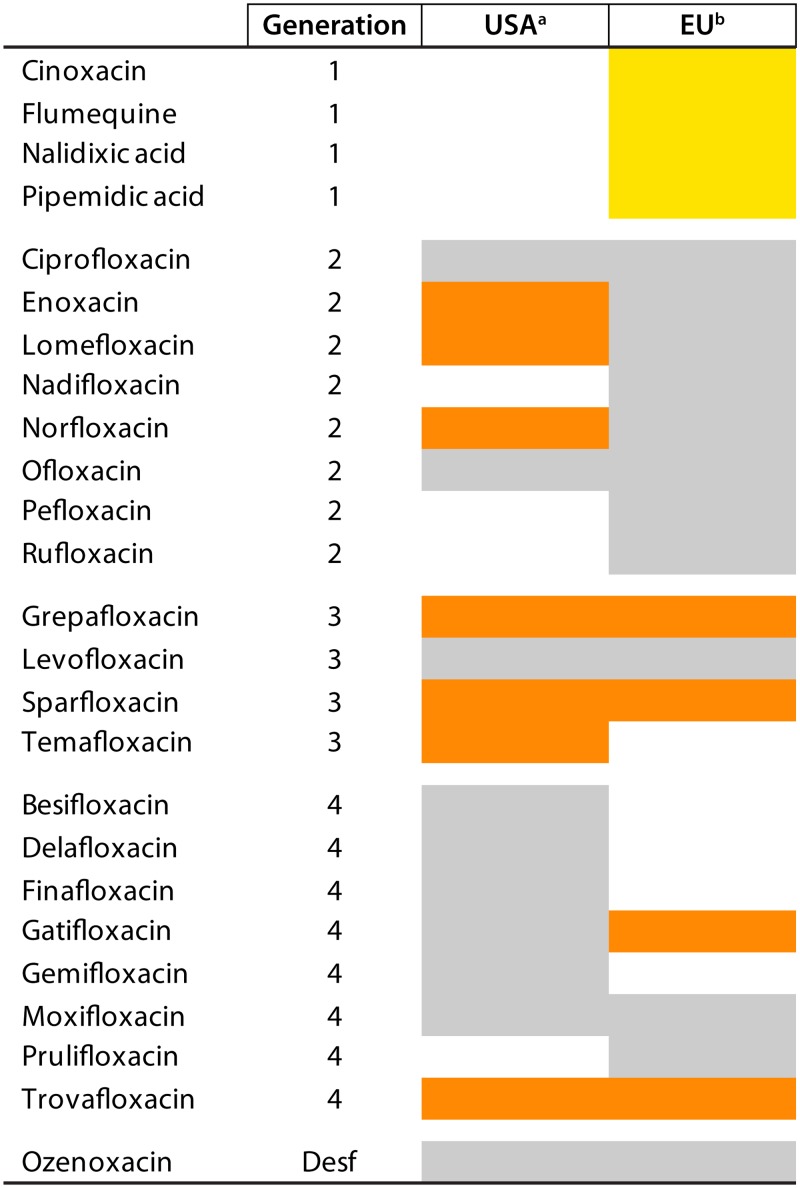
FIG 2.
Quinolones in use in human therapeutics in the United States and the European Union. In gray are quinolones currently (as of March 2019) used in human health. In orange are quinolones that have been discontinued (marked only when this information has been found). In yellow are quinolones proposed for withdrawal from use on March 2019 (https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-annex-i_en.pdf). Note that discontinuation may be related to adverse events (e.g., trovafloxacin) or to economical and market reasons. Some of these antibiotics (or other quinolones that have not been approved or are in the investigational phase) may be considered in special circumstances as last-resort treatment (454). Note that in all the cases, the data listed refer only to the United States and the European Union (including the United Kingdom at the time of writing). The introduction or current or past use/nonuse of these or other quinolones in other geographical areas may not be inferred by this figure. a, extracted from https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm; b, in use in at least one European Union member country (including the United Kingdom at the time of writing) (https://www.ema.europa.eu/documents/referral/quinolone-fluoroquinolone-article-31-referral-annex-i_en.pdf). G, generation; Desf, desfluoroquinolone.