To advance precision medicine and understanding of human health and disease, researchers, governments, private companies and patient groups are promoting the merits of collecting and sharing genetic, personal, environmental, and healthcare data on a massive scale (“biomedical big data”).1 Maximizing the utility of these data requires networks of comprehensive data resources for both research and clinical purposes; these networks are referred to here as medical information commons (MICs). Although similar to population-based and disease-specific biobanks,2 MICs are intended to host a breadth of data types, use novel computational tools for data analytics,3 rely on large health care delivery systems and the information technology industry to efficiently collect and manage information,4 and focus on both research and clinical applications.5 MICs capitalize on the recent explosion in personal and health-related data sharing through the use of smart devices, Internet-based social networking sites, and personal sensor-enabled digital medicine.6 They may also embrace a participant-centric focus to data sharing, research, and clinical care.7
To create a sustainable and ethical data resource within an ecosystem of multiple data assets and stakeholders, MICs must develop data-sharing polices that reflect public input.8 Numerous studies have been conducted to explore public attitudes towards biobanks.9 Many of these studies rely on focus groups and surveys, which collect opinions or assess initial responses. Those methodologies are valuable, but they do not incorporate perspectives based on well-developed, robust exploration of the issues and the value-laden trade-offs inherent in data-sharing policies. Public deliberation informed by democratic theory is one approach that can help gather informed public recommendations on value-laden issues, such as the widespread use of genetic data, by fostering a robust dialogue among deliberants.10
There have been some public deliberations regarding biobanks and genomic research, primarily in English-speaking countries (e.g., Canada, Australia, the U.K. and the U.S.) and often focused on specific biobanks with vested interests in the outcome. However, public input on building MICs has not been specifically addressed in prior deliberations.11 In addition, prominent recent examples of personal data breaches or misuse by public companies create a climate of suspicion among potential participants and makes successful MICs less likely without public accountability.12 We adopted a diverse, multi-site approach to elicit informed public recommendations regarding MIC design and management to help guide policymakers and other stakeholders.
Materials and Methods
We conducted public deliberations in three U.S. locations — (1) Durham, North Carolina, (2) Austin, Texas, and (3) Oakland, California — to obtain informed public input about the key issues policymakers must take into account before people are invited to share their personal, genetic, and health information with an MIC. Our approach to public deliberation was modeled on the Citizens’ Panel method, which involves in-person, active facilitation conducted over several days, and is best suited for more complex topics, such as data sharing, based on results from a randomized trial.13 The design of the deliberative panel (renamed “Community Advisory Panel” (CAP) to avoid potential sensitivities about citizenship status) reflected the efforts of the project team over several months and included engagement with experts in public deliberation, ethics, law, and policy. An MIC was defined for deliberants as “a virtual space where genetic, health, and other related information are stored, linked together, and shared electronically for the purposes of generating new knowledge through research, promoting public health, and improving the delivery of health care.”
In developing the questions that were posed and educational materials provided to deliberants, we drew on an extensive landscape review that included an assessment of over 300 existing MIC models and qualitative interviews with expert stakeholders to identify current challenges and promising new approaches to data sharing in an MIC.14 We cognitively tested and refined participant-facing materials to promote understanding;15 we also conducted a four-hour pilot deliberation in NC to improve the facilitation plan. These activities helped to inform the development of the final, overarching question posed to CAP deliberants: To represent the public’s values and interests, what issues should policymakers take into account when personal, genetic and health information is shared with a medical information commons?
To further elucidate public preferences, specific questions addressing five key ethical uncertainties were posed: (1) What type of permission, if any, should be required of people before their information is shared?; (2) What uses of the information should be encouraged or forbidden?; (3) What should people expect in return for sharing their information? What obligations, if any, should an MIC have to its participants?; (4) What role should the public play in governing an MIC? What key governance features should be in place to make an MIC trustworthy?; (5) What are your hopes and concerns for an MIC?
Recruitment
Although face-to-face public deliberations cannot fully represent the U.S. population, we sought to recruit deliberants from diverse geographic areas of the country, to capture regional differences in culture, politics, racial and ethnic balance, and educational climate. Our goal was to recruit a sufficiently wide range of “mini-publics” for the CAPs to be democratic, while keeping each CAP small enough in size to be genuinely deliberative.16
A professional recruitment firm recruited 30 adults age 18 or older in each location, with the aim of achieving 25 deliberants per CAP. The recruitment firm used a variety of recruitment tactics including online ads on Craigslist.org, Facebook, Reddit, and Next-Door and flyers posted at local libraries, community centers, and senior centers. Individuals were screened for eligibility on age, and then we purposefully sampled to achieve diversity in gender, race/ethnicity and educational attainment. We also purposefully included individuals with chronic illness since over time, many people with chronic diseases will likely be asked to share data in an MIC. Deliberants received compensation that varied depending on the local cost of living, ranging from $325 in Durham to $400 in Oakland. This project was deemed low-risk by the American Institutes for Research Institutional Review Board based on the conclusion that the CAPs involved the use of educational tests, survey procedures, interview procedures or observation of public behavior. When deliberants registered at each CAP site, they were asked to sign a media release form granting permission to record and videotape the session for use in producing written summaries of the deliberations.
Design and Implementation
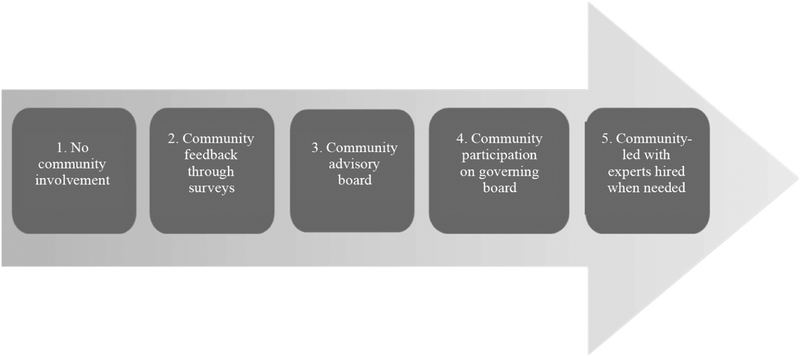
Each CAP took place over two days (Saturday and Sunday) between May and September 2017. Two weeks prior to the deliberative panel, deliberants were emailed an informational booklet and deliberants in Austin and Oakland were provided a link to a 30-minute educational video designed to provide an overview and basic explication of genetic, health, and personal data sharing and the potential benefits and harms of MICs. The booklet and video also described deliberants’ role in the deliberation, the importance of speaking on behalf of their community, and how findings would be shared.17 All background materials are available from the authors upon request. Each panel began with a 1-hour educational session to review information shared prior to the CAP. Hypothetical case studies (see Appendix A) developed by the project team stimulated discussions of specific topics first in small sub-groups followed by large group discussions, both moderated by facilitators. For example, through a case study modeled on the “All of Us” initiative,18 deliberants debated the benefits and harms of sharing specific types of data or allowing particular types of data uses and data users. Furthermore, through a large group presentation of the structure and responsibilities of a hypothetical MIC governing board and discussion of potential roles for public involvement (Exhibit 1), deliberants considered the features that are essential to promote MIC trustworthiness, including the public’s role in governing an MIC. Other strategies employed to generate discussion included a 2-hour non-facilitated session, called “open space,” which gave deliberants time to discuss topics of their own choosing and brainstorm recommendations. Instant polling was used periodically over the two days, enhancing the discussion by requiring deliberants to anonymously vote on positions (agree/disagree/not sure) and to explain their reasoning.19 During discussions, deliberants were presented with descriptions of potential benefits (e.g., sense of altruism, obtaining personal or family information, compensation) to prompt dialogue regarding what deliberants might expect in return for sharing their data with an MIC. We did not require participants to reach consensus during discussions to maximize free-ranging conversation and encourage deliberants with minority viewpoints to express their perspectives.20 It became clear during interchanges that deliberants’ expectations of an MIC were also incentives to share data. Therefore, we use the term “expectations” and “incentives” interchangeably.
Exhibit 1.
Governance Continuum
To reinforce the notion of deliberants representing community — rather than individual interests and values — we invited deliberants to wear a pin representing their state as a metaphorical, yet tactile symbol of their role as community representative. Deliberants were invited to wear the pin at the start of the second case study. This allowed deliberants considerable time to deliberate prior to being asked to represent a community. Deliberants were asked to define “community” as broadly or as narrowly as they saw fit (e.g., geographic area, racial identity, etc.). Facilitators did not tell participants how to define “community,” and encouraged participants have a malleable definition of community and to contemplate issues from community perspectives that differed from their own. Deliberants were told to remind others to reassume the role of community representative if deliberants deviated from that role. Further, facilitators encouraged deliberants to learn from others, explain their views, and work with others to make recommendations that benefit diverse communities. Facilitators did this by reviewing, posting, and working with deliberants to develop additional, as necessary, inclusive guidelines to govern the deliberations and asking probing questions. We created a graffiti wall and encouraged deliberants to write down their hopes and concerns regarding MICs at any point during the deliberation as another tool to facilitate conversation. At specific times during the two days, facilitators read out the hopes and concerns written on the graffiti wall, asking deliberants for clarification and inviting discussion.
At the end of the second day of each CAP, deliberants used instant polling to vote on recommendations. Recommendations were initially developed by facilitators based on concepts that emerged during deliberants’ structured and un-structured conversations. Facilitators presented the synthesized recommendations to deliberants. Deliberants then discussed each recommendation in the large group and revised the recommendations as necessary to align with the group’s opinions. Deliberants then voted on the recommendations. The recommendations deliberants voted on were used to inform the final recommendations presented below.
Analysis
All deliberations were digitally recorded, professionally transcribed, and de-identified. Topical codes were developed using both inductive and deductive methods.21 Transcripts were coded independently by two analysts using NVivo Pro 11 qualitative data analysis software.22 Any differences in analysts’ coding was resolved by discussion with the analysis team.
Data synthesis and reporting followed approaches based in the qualitative methodologies of directed content analysis to identify concepts in the transcripts and grounded theory to understand, through iterative and comparative techniques, the relationships or hierarchies among these concepts.23 Memo writing and quantitative pattern analysis were used to note patterns and themes, as well as relations among concepts. A third analyst compared thematic findings to whole transcript summaries and other data collected during deliberations (e.g., instant polling data) to check findings, examine exceptions and probe explanations.
To develop succinct, actionable recommendations, analysts synthesized cross-cutting themes and compared them to recommendations that emerged from voting sessions in each CAP. Two deliberants from each of the three CAPs volunteered to review and edit the recommendations reported in this paper. These six deliberants provided feedback via webinar and joined a March 2018 in-person meeting, where they were asked to ratify final recommendations and present them to the project’s Advisory Committee, which includes experts in ethics, law, and health policy. These recommendations were written as normative statements and were not ranked.
Deliberative Outcomes
A total of 75 people participated in the CAPs.24 They represented a range of demographic characteristics, with broad representation of race/ethnicity and levels of education (Table 1). Deliberants were predominately female (61%), racially diverse (63% non-white), middle-aged (mean 43 years), and had less than a college degree (75%). Approximately one-third of deliberants self-reported a chronic medical condition such as diabetes, hypertension, or allergies.
Table 1.
Deliberant Demographics45
| North Carolina | Texas | California | Total | |
|---|---|---|---|---|
| No. Deliberants | 27 | 26 | 22 | 75 |
| Gender (n, %) | ||||
| Male | l0 (37%) | 9 (35%) | l0 (45%) | 29 (39%) |
| Race/Ethnicity (n, %) | ||||
| Black/African American | 10 (37%) | 4 (l5%) | 6 (27%) | 20 (27%) |
| White | 12 (44%) | 11 (42%) | 5 (23%) | 28 (37%) |
| Age (Mean, SD) | 41 (15.60) | 44 (15.40) | 45 (15.46) | 43 (15.39) |
| Education (n, %) | ||||
| Technical/Vocational | 4 (15%) | 4 (15%) | 0 (0%) | 8 (11%) |
| Bachelor’s degree | 4 (15%) | 5 (19%) | 2 (9%) | 11 (15%) |
| Poverty Level (n, %) | ||||
| Chronic Condition (n, %) | ||||
| Children46 (n, %) | ||||
| No | 11 (41%) | l2 (46%) | 10 (48%) | 33 (45%) |
| Insurance Status (n, %) | ||||
| Uninsured | 6 (22%) | 4 (15%) | 3 (14%) | 13 (17%) |
| Experience with Genetic Tests (n, %) | ||||
| No | 15 (56%) | 17 (65%) | 15 (68%) | 47 (63%) |
Deliberants had nuanced, respectful dialogues regarding competing interests in sharing data with an MIC. Although most CAP deliberants strongly supported the concept of an MIC, many expressed concerns about its operations and how the data could be used. Value trade-offs are illustrated in themes that emerged from the CAP discussions, organized below by deliberative sub-question and corresponding recommendation(s).
1. What type of permission, if any, should be required from people before their information is shared? RECOMMENDATION:
The MIC informed consent process should be ongoing and not limited to one-time, blanket consent. As part of the ongoing consent process, participants should be able to decide what data they contribute to an MIC and how those data are used.
Deliberants expressed strong opposition to opt-out consent, calling it “sneaky” and “trickery,” and instead preferred an opt-in consent process through which people explicitly provide their permission to share data. The deliberants agreed that consent documentation should clearly state, in plain language, the purpose of an MIC, the types of data uses considered permissible, and possible benefits and harms of sharing data with researchers and other users.
Deliberants recognized that offering detailed information or one-on-one conversations to enable fully informed permission and data sharing authorization would be costly. However, deliberants described this cost as a necessary trade-off to help people understand the benefits and risks of sharing information and to optimize trust in an MIC: “Here you go, my information, just make sure that you keep it transparent. Tell me everything that it is that you need me to do. Tell me every single step that you’re gonna do. I know it’s costly, I know it’s time inefficient, but I still don’t want my information to be used for the wrong purpose.” [CA]
Most deliberants rejected broad consent, and they were divided regarding whether they wanted to provide consent each time an MIC received a request for data about them (study-specific consent). Some deliberants wanted to assess the risks and benefits for each proposed use of their data. Others preferred to learn about ways their data might be used before opting in, which could include indicating individual preferences governing categories of future data uses (categorical consent). Deliberants expressed support for offering the option of specific or categorical consent, with the additional possibility of changing one’s preferences over time (dynamic consent model): “Give them that option … because some people want to be able to say … exactly which ones [they consent to]. Some people are like, ‘Whatever, I want to use it for research, cool.’ But it gives them that option.” [TX]
2. What uses of the information should be encouraged or forbidden? RECOMMENDATION:
An MIC should set policies that prioritize data uses that serve the common good, particularly those that address major health issues in the U.S.
Across all CAP sites, how deliberants’ understood the proposed use of specific data types was an important factor in discussions and decisions about what data to share and with whom. Deliberants generally supported MIC data uses that have a clear connection to health-related research (e.g., studies on asthma rates in different neighborhoods or to develop tests to target chemotherapy for subgroups of cancer patients). Deliberants agreed that information such as medical records, environmental data, and daily exercise tracking data should be collected and shared because of a collective perceived benefit to health research.
Deliberants had mixed views on sharing DNA data. They acknowledged the key importance of DNA to medical advances, such as precision medicine, but raised serious concerns about illegitimate uses (e.g., cloning), stigmatization (e.g., of disease or racial groups), or discrimination (e.g., by law enforcement or insurers). Some deliberants expressed discomfort that their DNA information would be turned into a commodity. For example, one participant wondered if it might be possible “… for a retailer, to find out who you are by use of your DNA and then devise a way to discover what you bought or what you touched or where you went or who you knew.” [TX]
It was not evident to deliberants how some other types of data could be used for health-related research. Internet activity data were considered “too personal” and even when health-related aims were offered, most deliberants were skeptical that Internet data were an appropriate or reliable data source for research. Use of Internet activity data also sparked deep concerns about privacy, with some deliberants worrying that researchers might access other personal information, such as banking data.
There was broad, consistent support for MIC data use by university-based researchers because deliberants associated academic institutions with science and research. Conversely, deliberants strongly opposed use by law enforcement officials, saying they lack a direct connection to health-related research and might access MIC data (particularly DNA information) to unfairly target vulnerable individuals such as immigrants and minorities. Nevertheless, some deliberants indicated they could accept some law enforcement uses in service of the good of the community. Examples included identifying dangerous criminals, preventing serious crimes such as terrorism, or using data to improve police practices to make them more fair or sensitive to people’s medical conditions (e.g., mental illness).
When discussing for-profit users such as biopharmaceutical, health insurance, or technology companies, deliberants noted trade-offs between the benefit of potential medical advances and the risk of data being used for marketing purposes or to raise prices unfairly. While accepting that profit motivation could stimulate medical discoveries and product innovation, deliberants also noted that it is unacceptable for companies to make exorbitant profits when using voluntarily shared data. Deliberants were particularly skeptical of technology companies, citing concerns about lack of regulatory oversight and profit motives incentivizing non-health-related data uses. Also, while deliberants were informed that federal law protects health information and prohibits health insurers and many employers from discriminating against a person based on their genetics, deliberants expressed mistrust of health insurance companies’ motives. Deliberants assumed that health insurance companies would use MIC data to restrict or deny coverage, or to charge additional premiums.
The team presented deliberants with a set of hypothetical data uses such as pursuing an addiction gene, using exercise tracker data to qualify individuals for health insurance discounts, and using social media data to identify a link with depression. None of these was met with universal disapproval. For example, despite strong negative reactions to sharing social media data, deliberants struggled to reject the social media and depression research, noting that it could be beneficial to understand links between depression and social media use.
Finally, initial participant reactions to sharing data to help develop drugs for people in another country were mixed. Some deliberants felt that international data sharing was consistent with the United States’ commitment to helping others in the world, while others stated that MIC data uses should be limited to serving national interests. When the benefits of international data sharing to Americans were illustrated (e.g., studying rare disorders or infectious diseases containment), there was greater participant support, however this topic was not discussed in depth by most deliberants.
3. What should people expect in return for sharing their information? What obligations, if any, should an MIC have to its participants? RECOMMENDATION:
An MIC should compensate individuals as a matter of fairness, to enhance participation, and to ensure that an MIC represents the full diversity of the public. In addition, an MIC should have comprehensive rules for data integrity and security and enact strict penalties for data breaches or misuse.
Many deliberants expressed their expectation that people will share their data because it is “the right thing to do” to help others and serve the common good. Deliberants recognized the need for research or sharing of clinical data in support of the common good, expressing a moral duty to help their community or future generations. One participant explained that, “My information can be used to help people, save people’s lives, contribute to a medical breakthrough, I think it’s my moral responsibility to do that.” [CA] Although deliberants said that helping others could be reason enough to share their data, they also mentioned other societal benefits, such as monitoring disease outbreaks and saving public money as additional incentives. Deliberants noted ways in which receiving personal information from MICs could potentially benefit them directly, by informing them about their medical conditions, helping them choose treatments based on their DNA, or more easily sharing information with their doctors.
Deliberants also suggested that an MIC should offer to compensate people who share their data, to lessen the burden on those who have financial and/or logistical barriers to offering their time. Deliberants noted that compensation could take many forms — money, gift cards, donations to designated charities, or free or discounted medical treatments. Regardless of the form, most deliberants agreed that compensation was an incentive for individuals to enroll and sustain participation in an MIC. One participant explained, “We all struggle, so without that initial compensation, a lot of people don’t want to do it ‘cause they have kids to feed so they’re like, why would [I] stay an extra 20 minutes doing this when I have to go home, make dinner, do laundry….” [CA]
Deliberants also described compensation as a way for an MIC to give back to communities that have been unjustly exploited in past research. For example, deliberants referred to Henrietta Lacks whose family “… didn’t see a dime …” [NC] despite her immense contribution to science.25 Furthermore, deliberants expressed frustration that pharmaceutical and other health care companies often profit by using data they did not pay for by using MIC data to develop and sell new products. Deliberants noted that many communities distrust the health care system, medical researchers, and organizations that make money from health-related data. Accordingly, engaging these communities in data sharing may depend on adequate compensation.
Deliberants acknowledged potential drawbacks to compensation, such as increased cost of MIC operation. They also worried that offering financial compensation might only attract certain types of participants (e.g., those motivated by money instead of helping others), so that compensation could result in an MIC that is not fully representative and therefore would not benefit everyone equally.
With respect to MIC obligations, deliberants desired a large, inclusive MIC that attracts diverse groups of people so that research could benefit everyone. Furthermore, since deliberants feared that MIC data could be used to discriminate against people (e.g. by charging individuals with costly health conditions more for health insurance or denying them jobs), they noted that MIC obligations should include strong data security and anti-discrimination protections to avoid data misuse and minimize bias against certain groups of people (e.g., racial and ethnic minorities and individuals with mental illness). The protections should span data collection, storage, use, and disposal and there should be substantial, meaningful penalties for individuals and organizations that knowingly break the rules. These consequences should significantly affect the user’s reputation and financial standing.
4. What role should the public play in governing an MIC? What key governance features should be in place to make an MIC trustworthy? RECOMMENDATION:
To earn the public’s trust, an MIC should include diverse community representation in its governance structures, such as governing boards and committees. In addition, an MIC should have transparent governance practices, including plain language descriptions about how privacy will be ensured and how board members will be selected. MIC governance practices should also protect individuals and communities against discriminatory data uses, with regular auditing to ensure compliance by data users.
Deliberants stated that effective public representation in MIC governance was essential to promote public trust. The majority advocated for public representatives on governing boards, and for public representatives to have equal voting rights with experts. Importantly, deliberants noted that these public representatives must reflect the values and beliefs of their community. Deliberants recommended strategies such as convening town halls or collecting survey data to ensure that public representatives reflect the interests and values of their entire community. However, deliberants also expressed concerns that public representatives could be financially or politically motivated and/or that those selected may not have the technical expertise to participate effectively in making decisions. Additionally, deliberants wanted a transparent process for selecting public and expert representatives to serve on an MIC’s board. Without transparency, deliberants worried that outside groups could unduly influence MIC decisions regarding selection process.
Deliberants wanted an MIC governing board to restrict access to individuals or organizations meeting pre-established requirements for permissible uses. Even with this oversight and description of the Genetic Information Nondiscrimination Act (GINA) protections provided in background materials,26 deliberants worried that data could be misused, such as to discriminate against people with certain medical conditions: “If you were hiring someone for a job and you knew that somebody was more likely to die young maybe you wouldn’t want to hire them.” [NC]
Deliberants suggested MIC governance should monitor and audit data users to ensure they comply with MIC policies. Furthermore, deliberants thought there should be ways for individuals to report suspected data fraud, misuse, and unauthorized access. One participant explained that, “… You can bet that this information will be stolen, that it will be misused, that it will be used to gain profit for people who sell drugs and medical. So, my issue would be how, what sort of a process could be put in place for people who feel that they have been victimized by the misuse of this information, how they can bring that concern into a legal board?” [TX] Deliberants also recommended that MIC governance notify people when data breaches or misuse occurs.
5. What are your hopes and concerns for an MIC? RECOMMENDATION:
An MIC should ensure that the benefits and harms of an MIC are shared equitably.
Deliberants grappled with the trade-offs inherent in their hopes and concerns (Table 2), at times noting that specific considerations caused them to change their views. For example, some deliberants were opposed to sharing specific types of information with an MIC until they considered how that information might benefit medical research or society. Furthermore, deliberants paired discussions about potential MIC benefits they hoped for (e.g., medical advances) with the need for safeguards to protect deliberants from harm (e.g., data breaches and discrimination). Ultimately, most deliberants concluded that the potential for societal benefit outweighed the potential for harms, provided steps are taken by the MIC governing board to minimize harms.
Table 2.
Deliberant Hopes and Concerns
| Hopes | Concerns |
|---|---|
| Benefit future generations | Breaches of data security and inability to protect individuals’ privacy |
| Lead to ground-breaking medical advances (e.g. cure for cancer) | Accuracy and completeness of MIC data |
| Use for public health initiatives (e.g. monitoring disease outbreaks) | Interest groups and entities with money that could unduly influence MIC decisions concerning data uses |
| Result in health care cost savings | Unequal distribution of benefits resulting from a lack of diversity in participation |
| Provide participants information about their own health | Discriminatory uses of health information by employers or insurers |
| Ensure individual control of data uses, including opt-in consent process | Burdensome requirements, pressure or even coercion for participants to share their information allowed by MIC governing board |
| Require diverse community representation that would represent the publics interests | Stigmatization of individuals or communities |
| Offer direct benefits/incentives to deliberants when possible | People would be charged for accessing their information |
Discussion
Three CAPs were held in 2017 in different regions of the U.S. to deliberate policy-relevant issues regarding MIC data sharing, use and governance. In the background materials and case studies, specific efforts were made to highlight the MIC virtual data ecosystem, an analogy that emphasized the research and clinical applications of a broad range of health-relevant data produced in different settings, as well as the extensive number of ecosystem stakeholders. The results of this deliberation both reinforce findings from previous biobank public engagements and illustrate important differences for MICs.
First, deliberants strongly supported an MIC for medical- and health-related purposes in service of the greater good. This finding is consistent with other deliberations conducted in primarily white, highly educated groups as well as more diverse groups.27 In addition, CAP deliberants specifically recommended MIC policies that ensured prioritizing societal benefits over for-profit company interests, as well as prohibitions for uses such as marketing and law enforcement. Similar to previous studies,28 deliberants thought individuals providing data to MICs should be asked to provide explicit permission to sharing their data (opt-in rather than opt-out informed consent) and maintain some individual control over data uses. There was little support for broad consent (for unspecified future uses) and a preference for exercising additional control through consent models such as categorical, study-specific and dynamic consent.
As has been shown in other deliberations,29 MIC access by commercial interests, such as biopharmaceutical companies, reduces participant trust and willingness to share data. While recognizing that commercial involvement is inevitable (and sometimes required for treatment advances), deliberants described the need for governance structures that protect against disproportionate influence by private sector funders and data users. These findings are reinforced by participant attitudes towards sharing medical claims data and Internet activity data — both were viewed less favorably by deliberants than other types of data. Negative deliberant attitudes towards sharing Internet and social media data are similar to findings from a recent national survey about willingness to share data with the Precision Medicine Initiative, that also documented the public’s reluctance to share these data.30 Deliberants had difficulty seeing the relevance of Internet and social media data to health-related research or clinical care, but also thought these data types were “too personal” to be shared with an MIC.
Consistent with other biobank-related public deliberations,31 deliberants expected robust data security and the protection of privacy as a fundamental condition for sharing data with an MIC. They greatly valued data security and privacy protections to prevent data breaches in an environment characterized by exponential growth in data volume. One deliberant suggested utilizing novel information technologies such as block chain and this idea was supported by others in his CAP.32 In keeping with the data security framework advanced by the White House regarding the Precision Medicine Initiative, deliberants recommended establishing policies, procedures, and independent audits to protect against data misuses.33 However, MIC deliberants went a step further to recommend substantial, meaningful penalties for individuals and organizations that knowingly break the rules, suggesting that sanctioning only researchers was an inadequate deterrent for powerful corporate interests.
Our CAPs were distinctive from previous biobank-focused public deliberations in several important ways. Although population-based biobanks are built on the premise that their data collections should represent the full diversity of the population,34 most public deliberations have not included minority groups and individuals with lower levels of educational attainment to the degree achieved in this project. Deliberants expressed a strong desire for an MIC that includes and serves the full diversity of the population and their health care interests and needs. They expressed concern that external influencers of an MIC, such as institutional bias, commercial interests, or financial constraints of the designers, might lead to the exclusion of some types of people, communities, or health-related information from an MIC. Therefore, deliberants recommended that an MIC should ensure that its benefits reach all members of the community, including those who have historically been discriminated against because of race, ethnicity, or socioeconomic status. To achieve the desired diversity of community participation, deliberants endorsed policies supporting compensation for sharing data as an incentive. Although altruism was a strong motivator for many deliberants, compensation was viewed as a necessary strategy to overcome barriers to enrollment and sustained participation for disadvantaged groups. This finding contrasts with other public deliberations that found little or no support for compensating individuals, citing concerns regarding unfairly encouraging participation.35
Deliberants also worried that data in an MIC could be used to unfairly target or penalize groups of people such as racial minorities, rather than benefit them. Within particular CAP sites and local communities, we observed the legacy of historical betrayals of trust, leading to persistent distrust of researchers and institutions. Therefore, building trust, as well as being aware of and addressing distrust, is critical for MIC designers and managers.
The current project advances understanding of how to structure public participation in governance by specifically recommending inclusion of community representatives with equal voting rights on MIC governing boards. Deliberants also endorsed transparent procedures for selecting community representatives and modest compensation to cover participation costs. However, deliberants cautioned against paying community members at the same level as experts, to avoid the possibility of monetary payments becoming the prime motivator for participation on the governing board. In addition, deliberants agreed that public representatives had an obligation to reflect the broad interests of their community and should conduct surveys or town halls to better reflect community priorities.
Although CAP deliberants hoped that sharing their information with an MIC would help bring about medical advances, their substantial concerns reflect a sense of loss of control over their data and a lack of trust in institutions and private sector companies to ensure privacy and protect against data misuse, a trend that has been observed nationally.36 During the deliberations, deliberants described their frustration with the fact that they have little control over or understanding of how their individual data are being collected, shared, and used in other contexts such as social media, financial transactions, and internet searches. Deliberants’ frustration with their general lack of control over their own data and negative experiences with medical record accuracy contributed to a general mistrust of institutions.
Trust has been identified as an important predictor of willingness to participate in a biobank.37 However, recent assessments of trust in biobanks reveal that, as the boundaries between research and practice become more fluid, trust becomes a multi-dimensional construct between trustor, trustee, and the particular data-sharing context.38 We observed this complexity as part of the CAP recommendations, suggesting that a trustworthy MIC will need directly to address the full burden of the public’s mistrust of medical research and the larger health care system in general. The CAP deliberations also aligned with a recent conceptualization of trust in the precision medicine context that emphasizes the need for trust facilitators.39 Specifically, trust facilitators endorsed by deliberants included technological innovations (to ensure data security and minimize risk of re-identifying MIC data), governance practices that explicitly address the public’s desire for transparency, respect for autonomy through opt in permission, and participant engagement.40 To achieve these goals will require ongoing public education regarding data sharing trade-offs in both research and health care delivery, as well as respect for and attention to the diverse sources of community mistrust.
Limitations
Although we recruited racially and socioeconomically diverse deliberants, we included only English-speaking deliberant groups, therefore our results may not reflect interests and values of non-English speakers. Also, the topic of MICs is complex and given that each group met only for two days, some deliberants felt there was not enough time to fully address each of the topics during the sessions. We also do not know whether their views as expressed would be sustained or would change if another meeting were held a few months later.41
There is also the possibility of recruitment bias given the recruitment firm’s reliance on social media sites to locate potential deliberants. While attempts were made to diversify recruitment strategies by posting flyers in public venues such as libraries and senior centers, deliberants were primarily recruited online. While a review of health policy research and public health deliberations found that the most commonly used recruitment method involved a professional recruiter or market research company,42 other deliberations regarding biobanks have stressed the value of random digit dialed recruitment stratified for demographic categories derived from census data.43 We attempted to achieve a balance of these two approaches, providing the professional recruiter with recruitment targets based on regional census data. The impact of recruitment bias among CAP deliberants versus the general population is most likely modest as currently the majority of U.S. adults access social media sites.44
Finally, because we did not require deliberants to reach consensus in an effort to maximize free-ranging discussion, policy recommendations were developed by combining individual CAP recommendations and deliberations. Although we obtained final review and approval from six CAP representatives, not all deliberants voted for these recommendations. Nevertheless, the iterative process used to develop the final recommendations ensures that the sentiments of the deliberants are captured in the final recommendations.
Conclusion
An ecosystem of biomedical big data organized as an MIC holds great potential for both research and clinical care, but also requires data contributed voluntarily by individuals with diverse community affiliations. The fulfillment and sustainability of this ecosystem is dependent on participant engagement and trust. Recommendations from CAPs, based on public engagement informed by theories of deliberative democracy, demonstrate how MIC stakeholders can develop policies that recognize and account for high levels of public mistrust, while also catalyzing socially responsible advancement of precision medicine.
Supplementary Material
An ecosystem of biomedical big data organized as an MIC holds great potential for both research and clinical care, but also requires data contributed voluntarily by individuals with diverse community affiliations. The fulfillment and sustainability of this ecosystem is dependent on participant engagement and trust. Recommendations from CAPs, based on public engagement informed by theories of deliberative democracy, demonstrate how MIC stakeholders can develop policies that recognize and account for high levels of public mistrust, while also catalyzing socially responsible advancement of precision medicine.
Acknowledgements
The deliberants in all three Community Advisory Panels (CAP) are gratefully acknowledged. In particular, we recognize the contributions of the six CAP deliberants who attended an in-person meeting with the project’s Advisory Committee to represent their cohort and present CAP recommendations. Similarly, we are indebted to our collaborators (L. Downing, D. Ganachari, M. Maurer, and E. Pathak-Sen) for providing advice and guidance in the design and conduct of the deliberative exercise. The work presented in this article is funded by the National Institutes of Health (NIH), National Human Genome Research Institute grant R01 HG008918 (AMG and RCD). The views expressed in this article are those of the authors and do not necessarily reflect those of the NIH.
Footnotes
Dr. McGuire reports personal fees from Geisinger Research, outside the submitted work.
References
- 1.National Research Council, 2011, Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease (Washington, DC: The National Academies Press; ); [PubMed] [Google Scholar]; Topol EJ, “The Big Medical Data Miss: Challenges in Establishing an Open Medical Resource,” Nature Reviews Genetics 16, no. 5 (2015): 253–254, available at < 10.1038/nrg3943> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; LunaDNA, LunaDNA, Home Page, available at <http://www.lunadna.com>. (last visited July 25, 2018);; UK Biobank, UK Biobank, Home Page, available at <http://www.ukbiobank.ac.uk> (last visited July 25, 2018);; CARTaGENE, CARTaGENE, Home Page, available at <http://www.cartagene.qc.ca/en/home> (last visited July 25, 2018);; deCODE genetics, Unrivaled capabilities, Science, available at <http://www.decode.com/research> (last visited July 25, 2018);; Terry SF, Shelton R, Biggers G, Baker D, and Edwards K, “The Haystack is Made of Needles,” Genetic Testing and Molecular Biomarkers 17, no. 3 (2013): 175–177, available at < 10.1089/gtmb.2012.1542> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Vayena E and Blasimme A, “Biomedical Big Data: New Models of Control Over Access, Use and Governance,” Bioethical Inquiry 14, no. 4 (2017): 501–513, available at < 10.1007/s11673-017-9809-6> (last visited March 1, 2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biobanks in this context are repositories of human biological material (e.g., DNA, RNA, tissue, organs, cells) and associated data that can be used in many types of research.
- 3.Adams SA and Petersen C, “Precision Medicine: Opportunities, Possibilities, and Challenges for Patients and Providers,” Journal of the American Medical Informatics Association 23, no. 4 (2016): 787–790, available at < 10.1093/jamia/ocv215> (last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson MV, “Prevision Medicine at the Crossroads,” Human Genomics 11, no. 23 (2017), available at < 10.1186/s40246-017-0119-1> (last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deverka PA, Majumder MA, Villanueva AG, Anderson M, et al. , “Creating a Data Resource: What Will It Take to Build a Medical Information Commons?” Genome Medicine 9, no. 84 (2017): 1–5, available at <https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-017-0476-3> (last visited January 17, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elenko E, Underwoord L, and Zohar D, “Defining Digital Medicine,” Nature Biotechnology 33, no. 5 (2015): 456–461, available at < 10.1038/nbt.3222> (last visited March 1, 2019). [DOI] [PubMed] [Google Scholar]
- 7.Fleurence RL et al. , “Launching PCORnet, A National Patient-Centered Clinical Research Network,” Journal of the American Medical Informatics Association: JAMIA 21, no. 4 (2014): 578–582, available at < 10.1136/amiajnl-2014-002747> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Dwamena F et al. , “Interventions for Providers to Promote a Patient-Centred Approach in Clinical Consultations,” Cochrane Database of Systematic Reviews no. 12 (2012): CD003267, available at < 10.1002/14651858.CD003267.pub2> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Blasimme A and Effy V, “Becoming Partners, Retaining Autonomy: Ethical Considerations on the Development of Precision Medicine,” BMC Medical Ethics 17, no. 67 (2016), available at < 10.1186/s12910-016-0149-6> (last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.See P.A. Deverka, supra note 5.
- 9.Cambon-Thomsen A, Rial-Sebbag R, and Knoppers BM, “Trends in Ethical and Legal Frameworks for the use of Human Biobanks,” The European Respiratory Journal 30, no. 2 (2007): 373–382, available at < 10.1183/09031936.00165006> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Clayton EW, “Informed Consent and Biobanks,” The Journal of Law, Medicine & Ethics 33, no. 1 (2005): 15–21, available at < 10.1111/j.1748-720X.2005.tb00206.x> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Meslin EM and Cho MK, “Research Ethics in the Era of Personalized Medicine: Updating Science’s Contract with Society,” Public Health Genomics 13, no. 6 (2010): 378–384, available at < 10.1159/000319473> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, and Hudson K, “Public Perspectives on Informed Consent for Biobanking,” American Journal of Public Health 99, no. 12 (2009): 2128–2134, available at < 10.2105/AJPH.2008.157099> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Winickoff DE and Winickoff RN, “The Charitable Trust as a Model for Genomic Biobanks,” The New England Journal of Medicine 349, no. 12 (2003): 1180–1184, available at < 10.1056/NEJMsb030036> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, and Hudson K, “Public Expectations for Return of Results from Large-cohort Genetic Research,” American Journal of Bioethics 8, no. 11 (2008): 36–43, available at < 10.1080/15265160802513093> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Kaufman DJ, Murphy-Bollinger J, Scott J, and Hudson KL, “Public Opinion about the Importance of Privacy in Biobank Research,” American Journal of Human Genetics 85, no. 5 (2009): 643–654, available at < 10.1016/j.ajhg.2009.10.002> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Platt J et al. , “Public Preferences Regarding Informed Consent Models for Participation in Population-Based Genomic Research,” Genetics in Medicine 16, no. 1 (2014): 11–18, available at < 10.1038/gim.2013.59> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Pulley JM, Brace MM, Bernard GR, and Masys DR, “Attitudes and Perceptions of Patients Towards Methods of Establishing a DNA Biobank,” Cell Tissue Banking 9, no. 1 (2008): 55–65, available at < 10.1007/s10561-007-9051-2> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Garrison NA et al. , “A Systematic Literature Review of Individuals’ Perspectives on Broad Consent and Data Sharing in the United States,” Genetics in Medicine 18, no. 7 (2016): 663–671, available at < 10.1038/gim.2015.138> (last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abelson J et al. , “Deliberations about Deliberative Methods: Issues in the Design and Evaluation of Public Participation Processes,” Social Science & Medicine 57, no. 2 (2003): 239–251, available at < 10.1016/S0277-9536(02)00343-X> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Burkhalter S, Gastil J, and Kelshaw T, “A Conceptual Definition and Theoretical Model of Public Deliberation in Small Face-to-Face Groups,” Communications Theory 12, no. 4 (2002): 398–422, available at < 10.1111/j.1468-2885.2002.tb00276.x> (last visited March 1, 2019); [DOI] [Google Scholar]; Carman KL et al. , “Effectiveness of Public Deliberation Methods for Gathering Input on Issues in Healthcare: Results from a Randomized Trial,” Social Science & Medicine 133 (2015): 11–20, available at < 10.1016/j.socscimed.2015.03.024> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Delli Carpini MX, Cook FL, and Jacobs LR, “Public Deliberation, Discursive Participation, and Citizen Engagement: A Review of the Empirical Literature,” Annual Review of Political Science 7, no. 1 (2004): 315–344, available at < 10.1146/annurev.polisci.7.121003.091630> (last visited March 1, 2019); [DOI] [Google Scholar]; Hamlett PW, “Technology Theory and Deliberative Democracy,” Science, Technology, & Human Values 28, no. 1 (2003): 112–140, available at < 10.1177/0162243902238498> (last visited March 1, 2019). [DOI] [Google Scholar]
- 11.Lemke AA, Halverson C, and Ross LF, “Biobank Participation and Returning Research Results: Perspectives from a Deliberative Engagement in South Side Chicago,” American Journal of Medical Genetics Part A 158A, no. 5 (2012): 1029–1037, available at < 10.1002/ajmg.a.34414> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Molster C et al. , “An Australian Approach to the Policy Translation of Deliberated Citizen Perspectives on Biobanking,” Public Health Genomics 15 (2012): 82–91, available at < 10.1159/000334104> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Haddow G et al. , “Generation Scotland: Consulting Publics and Specialists at an Early Stage in a Genetic Database’s Development,” Critical Public Health 18, no. 2 (2008): 139–149, available at < 10.1080/09581590701824086> (last visited March 1, 2019); [DOI] [Google Scholar]; Lemke AA et al. , “Public and Biobank Participant Attitudes Toward Genetic Research Participation and Data Sharing,” Public Health Genomics 13, no. 6 (2010): 368–377, available at < 10.1159/000276767> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; O’Doherty KC and Burgess MM, “Engaging the Public on Biobanks: Outcomes of the BC Biobank Deliberation,” Public Health Genomics 12, no. 4 (2009): 203–215, available at < 10.1159/000167801> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Hicks A, Hellyers JH, and Koenig B, “Involving the Public in Planning for the Genomics Revolution: An Experiment in Deliberative Democracy,” The Bioethics Examiner 11, no. 2 (2008): 1–3, available at <https://www.ahc.umn.edu/img/assets/26102/BTHX_Examiner_08.pdf> (last visited February 26, 2019); [Google Scholar]; Gornick MC, Sherer AM, Sutton EJ, Ryan KA, Exe NL, Li M, Uhlmann WR, Kim SYH, Roberts JS, and DeVries RG, “Effect of Public Deliberation on Attitudes Toward Return of Secondary Results in Genomic Sequencing,” Journal of Genetic Counseling 26, no. 1 (2017): 122–132, available at < 10.1007/s10897-016-9987-0> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Dry SM et al. , “Community Recommendations on Biobank Governance: Results from a Deliberative Community Engagement in California,” PLoS One 12, no. 2 (2017): e0172582, available at < 10.1371/journal.pone.0172582> (last visited March 1, 2019); [DOI] [PMC free article] [PubMed] [Google Scholar]; Burgess M, O’Doherty K, and Secko D, “Biobanking in British Columbia: Discussions of the Future of Personalized Medicine Through Deliberative Public Engagement,” Personalized Medicine 5, no. 3 (2008): 285–296, available at < 10.2217/17410541.5.3.285> (last visited March 1, 2019); [DOI] [PubMed] [Google Scholar]; Nicol D et al. , “Understanding Public Reactions to Commercialization of Biobanks and use of Biobank Resources,” Social Science & Medicine 162 (2016): 79–87, available at < 10.1016/j.socscimed.2016.06.028> (last visited March 1, 2019). [DOI] [PubMed] [Google Scholar]
- 12.Bomey N et al. , “Equifax data breach: What you need to know about hacking crisis,” USA Today, September 15, 2017, available at <https://www.usatoday.com/story/money/2017/09/15/equifax-data-breach-what-you-need-know-hacking-crisis/670166001> (last visited October 24, 2018); [Google Scholar]; Fung B, “Equifax’s massive 2017 data breach keeps getting worse,” Washington Post, March 4, 2018, available at <https://www.washingtonpost.com/news/the-switch/wp/2018/03/01/equifax-keeps-finding-millions-more-people-who-were-affected-by-its-massive-data-breach/> (last visited October 24, 2018); [Google Scholar]; Badshah N, “Facebook to contact 87 million users affected by data breach,” Guardian (London), April 8, 2018, available at <https://www.theguardian.com/technology/2018/apr/08/facebook-to-contact-the-87-million-users-affected-by-data-breach> (last visited October 24, 2018); [Google Scholar]; Moise I, “Whole Foods Discloses Data Breach,” Wall Street Journal, September 28, 2017, available at <https://www.wsj.com/articles/whole-foods-discloses-data-breach-1506636659> (last visited October 24, 2018); [Google Scholar]; Shoot B, “Facebook Closes Loophole That Revealed Personal Data of People in ‘Closed’ Groups,” Fortune, July 12, 2018, available at <http://fortune.com/2018/07/12/facebook-chrome-extension-closed-group-loophole> (last visited October 24, 2018); [Google Scholar]; Matloff E, “Facebook Violates Trust of ‘Private’ Patient Groups,” Forbes, July 29, 2018, available at <https://www.forbes.com/sites/ellenmatloff/2018/07/29/facebook-violates-trust-of-private-patient-groups> (last visited October 24, 2018); [Google Scholar]; Brandom R, “Facebook Changes Privacy Settings after Outing Members of a Closed Medical Support Group,” The Verge, July 12, 2018, available at <https://www.theverge.com/2018/7/12/17565754/facebook-brca-private-group-breast-cancer> (last visited October 24, 2018); [Google Scholar]; Hensel A, “Marketers Could Harvest Member Data from Closed Facebook Groups as Recently as June, Report Says,” VentureBeat, July 12, 2018, available at <https://venturebeat.com/2018/07/12/marketers-could-harvest-member-data-from-closed-facebook-groups-as-recently-as-june-report-says> (last visited October 24, 2018); [Google Scholar]; Fazzini K, “Facebook Recently Closed a Loophole that Allowed Third Parties to Discover the Names of People in Private, ‘Closed’ Facebook Groups,” CNBC, July 12, 2018, available at <https://www.cnbc.com/2018/07/11/facebook-private-groups-breast-cancer-privacy-loophole.html> (last visited October 24, 2018); [Google Scholar]; Allen M, “Health Insurers Are Vacuuming Up Details About You — And It Could Raise Your Rates,” ProPublica, July 17, 2018, available at <https://www.propublica.org/article/health-insurers-are-vacuuming-up-details-about-you-and-it-could-raise-your-rates> (last visited October 24, 2018). [Google Scholar]
- 13.See K.L. Carman, supra note 10.
- 14.Villanueva AG, Cook-Deegan R, Koenig BA, Deverka PA et al. , “Characterizing the Biomedical Data-Sharing Landscape,” Journal of Law, Medicine & Ethics 47, no. 1 (2019): 21–30; [DOI] [PMC free article] [PubMed] [Google Scholar]; Majumder MA, Bollinger JM, Villanueva AG, and Deverka PA et al. , “The Role of Participants in a Medical Information Commons,” Journal of Law, Medicine & Ethics 47, no. 1 (2019): 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality, The Purpose and Process of Cognitive Testing, Quality and Patient Safety, available at <http://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/resources/cognitive/index.html> (last visited October 24, 2018). [Google Scholar]
- 16.Goodin RE and Dryzek JS, “Deliberative Impacts: The Macro-Political Uptake of Mini-Politics,” Politics & Society 34, no. 2 (2006): 219–244, available at < 10.1177/0032329206288152> (last visited March 1, 2019). [DOI] [Google Scholar]
- 17.The methods differed slightly by site as we learned by doing (e.g., Durham deliberants expressed the need for additional background information delivered in different formats. Therefore, we developed an educational video and made it available to Austin and Oakland deliberants both prior to and during the deliberations.)
- 18.National Institutes of Health, Home Page, All of Us, available at <https://www.allofus.nih.gov>. (last visited August 6, 2018). [Google Scholar]
- 19.Involve, People & Participation: How to Put Citizens at the Heart of Decisions-Making, National Coalition for Dialogue & Deliberation, available at <http://www.ncdd.org/exchange/files/docs/People_Participation.pdf> (last visited August 2, 2018); [Google Scholar]; National Coalition for Dialogue & Deliberation, Engagement Steams Framework, National Coalition for Dialogue & Deliberation, available at <http://www.ncdd.org/files/rc/2014_Engagement_Streams_Guide_Web.pdf> (last visited August 2, 2018); [Google Scholar]; Hofmaier C, “Open Space Technology,” Tools and instruments for a better societal engagement in “Horizon 2020,” Engage2020, 2014, available at <http://engage2020.eu/media/Fact-sheet-on-the-current-praxis-of-methods-tools-and-instruments.pdf> (last visited August 2, 2018); [Google Scholar]; Rowe G and Frewer LJ, “Typology of Public Engagement Mechanisms,” Science, Technology, & Human Values 30, no. 2 (2005): 251–290, available at < 10.1177/0162243904271724> (last visited March 1, 2019). [DOI] [Google Scholar]
- 20.Carman KL, Heeringa JW, Heil SKR, Garfinkel SG, Windham A, Gilmore D, Ginsburg M, Sofaer S, Gold M, and Pathak-Sen E, Public Deliberation To Elicit Input on Health Topics: Findings From a Literature Review, AHRQ, Publication No. 12-EHC070-EF (February 2013); [Google Scholar]; Friberg-Fernros H and Schaffer JK, “The Consensus Paradox: Does Deliberative Agreement Impede Rational Discourse?” Political Studies 62, no. 1 Supplement (2014): 99–116; [Google Scholar]; Solomon S and Abelson J, “Why and When Should We Use Public Deliberation?” The Hastings Center Report 42, no. 2 (2012): 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creswell JW and Poth C, Qualitative Inquiry and Research Design, 4th ed. (Thousand Oaks, CA: Sage, 2016); [Google Scholar]; Miles MB, Huberman A, and Saldana J, Qualitative Data Analysis, 3rd ed. (Thousand Oaks, CA: Sage, 2014). [Google Scholar]
- 22.NVivo qualitative analysis Software [Computer software], QSR International Pty Ltd., Version 10, 2012. [Google Scholar]
- 23.See J.W. Creswell, supra note 21.
- 24.Over the course of two days, four Oakland CAP deliberants left the deliberation early due to either family or work obligations; two left early because of perceived racial bias between deliberants.
- 25.Ravitz J, “Henrietta Lacks: Her Cells, her Legacy,” CNN, April 28, 2018, available at <https://www.cnn.com/2017/04/21/health/henrietta-lacks-legacy/index.html> (last visited October 24, 2018). [Google Scholar]
- 26.42 USC § 21F (2008).
- 27.See C. Molster et al., supra note 11.
- 28.See N.A. Garrison, supra note 9.
- 29.See S.M. Dry et al., supra note 11.
- 30.Kaufman DJ et al. , “A Survey of U.S Adults’ Opinions about Conduct of a Nationwide Precision Medicine Initiative Cohort Study of Genes and Environment,” PLoS One 11, no. 8 (2016): e0160461, available at < 10.1371/journal.pone.0160461> (last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.See S.M. Dry, supra note 11.
- 32.Gordon WJ and Catalini C, “Blockchain Technology for Healthcare: Facilitating the Transition to Patient-Driven Interoperability,” Computational and Structural Biotechnology Journal 16 (2018): 224–230, available at < 10.1016/j.csbj.2018.06.003> (last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institutes of Health, Achieving the Principles through a Prevision Medicine Initiative Data Security Policy Framework, All of Us, available at <https://www.allofus.nih.gov/about/program-overview/precision-medicine-initiative-data-security-policy-principles-and-framework-overview/achieving-principles-through-precision-medicine-initiative-data-security-policy-framework> (last visited August 6, 2018). [Google Scholar]
- 34.See S.M. Dry, supra note 11;; deCODE genetics, deCODE genetics, Home Page, available at <http://www.decode.com>. (last visited August 6, 2018);; University of Tartu Institutes of Genomics, Estonian Genome Center, Home Page, available at <https://www.geenivaramu.ee/en> (last visited August 6, 2018).
- 35.See C. Molster and K.C. O’Doherty, supra note 11.
- 36.Madden M, Rainie L, Zickuhr K, Duggan M, and Smith A, Public Perceptions of Privacy and Security in the Post-Snowden Era, Pew Research Center, available at <http://www.pewinternet.org/2014/11/12/public-privacy-perceptions/> (last visited August 6, 2018). [Google Scholar]
- 37.Critchley C, Nicol D, and Otlowski M, “The Impact of Commercialisation and Genetic Data Sharing Arrangements on Public Trust and the Intention to Participate in Biobank Research,” Public Health Genomics 18, no. 3 (2015): 160–172, available at < 10.1159/000375441> (last visited March 1, 2019). [DOI] [PubMed] [Google Scholar]
- 38.Platt J and Kardia S, “Public Trust in Health Information Sharing: Implications for Biobanking and Electronic Health Record Systems,” Journal of Personalized Medicine 5, no. 1 (2015): 3–21, available at < 10.3390/jpm5010003> (last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adjekum A, Ienca M, and Vayena E, “What Is Trust? Ethics and Risk Governance in Precision Medicine and Predictive Analytics,” OMICS: A Journal of Integrative Biology 21, no. 12 (2017): 704–710, available at < 10.1089/omi.2017.0156>(last visited March 1, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.See A. Adjekum, supra note 39.
- 41.See M.C. Gornick, supra note 11.
- 42.Degeling C, Carter SM, and Rychetnik L, “Which Public and Why Deliberate? – A Scoping Review of Public Deliberation in Public Health and Health Policy Research,” Social Science & Medicine 131 (2015): 114–121. [DOI] [PubMed] [Google Scholar]
- 43.Longstaff H and Burgess MM, “Recruiting for Representation in Public Deliberation on the Ethics of Biobanks,” Public Understanding of Science 19, no. 2 (2010): 212–224, available at < 10.1177/0963662508097626> (last visited March 1, 2019). [DOI] [PubMed] [Google Scholar]
- 44.Pew Research Center, Social Media Fact Sheet, Pew Research Center, available at <http://www.pewinternet.org/fact-sheet/social-media/> (last visited August 17, 2018). [Google Scholar]
- 45.Included are all deliberants who signed in at the start of the first day of deliberation.
- 46.One deliberant from California did not respond to this question. Therefore, percentages for California for this question are calculated using N = 21. The totals for this characteristic are calculated using N = 74.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.