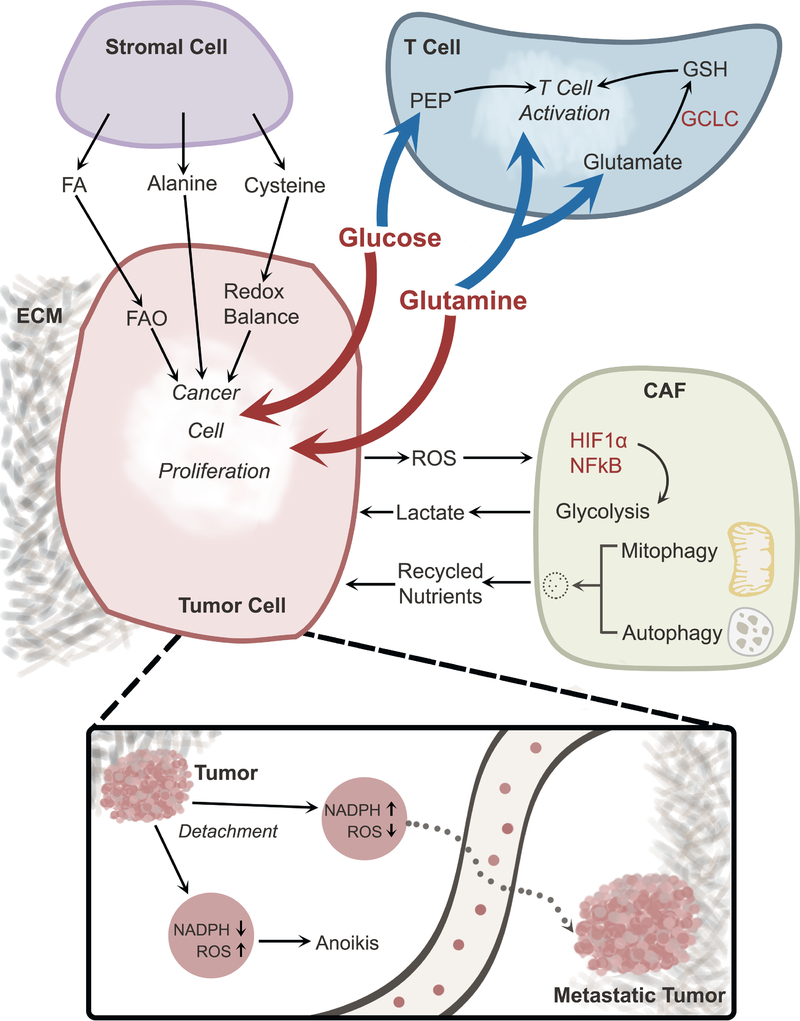
Figure 4. Metabolic cross-talk in the tumor microenvironment.
Interactions among cancer cells and other components of the TME contribute to metabolic heterogeneity. Cancer cells and T cells compete for nutrients (e.g., glucose and glutamine), and excessive consumption of these nutrients by cancer cells suppresses T cell activation. Other cells in the TME also engage in metabolic cross-talk with cancer cells. Stromal cells provide nutrients that support cancer cell proliferation. CAFs respond to oxidative stress imposed by cancer cells by activating HIF1α and NFκB, thereby stimulating glycolysis and secreting lactate, which may be taken up by cancer cells. Degradative processes like mitophagy and autophagy in CAFs also provide nutrients to cancer cells. Loss of ECM attachment suppresses NADPH production, resulting in ROS-mediated anoikis; this form of cell death can be overcome through oncogene-stimulated NADPH production by the pentose phosphate pathway. Reducing oxidative stress allows cells to survive in the detached state and promotes formation of distant metastases. Abbreviations, TME, tumor microenvironment; ECM, extracellular matrix; FA, fatty acid; FAO, fatty acid oxidation; CAF, cancer-associated fibroblast; ROS, reactive oxygen species; PEP, phosphoenolpyruvate; GSH, glutathione, reduced; GCLC, Glutamate-Cysteine Ligase.