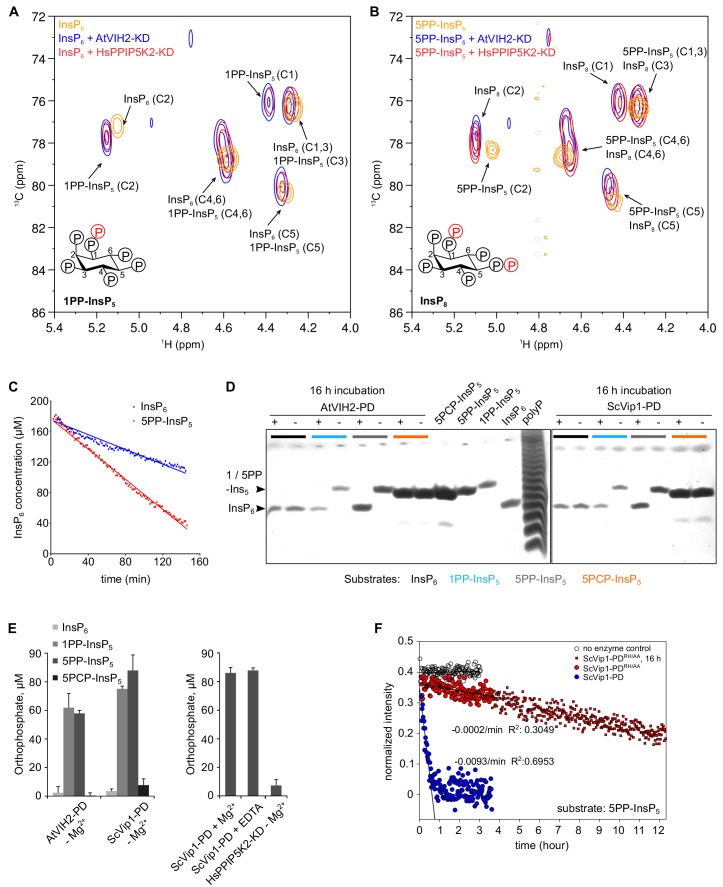
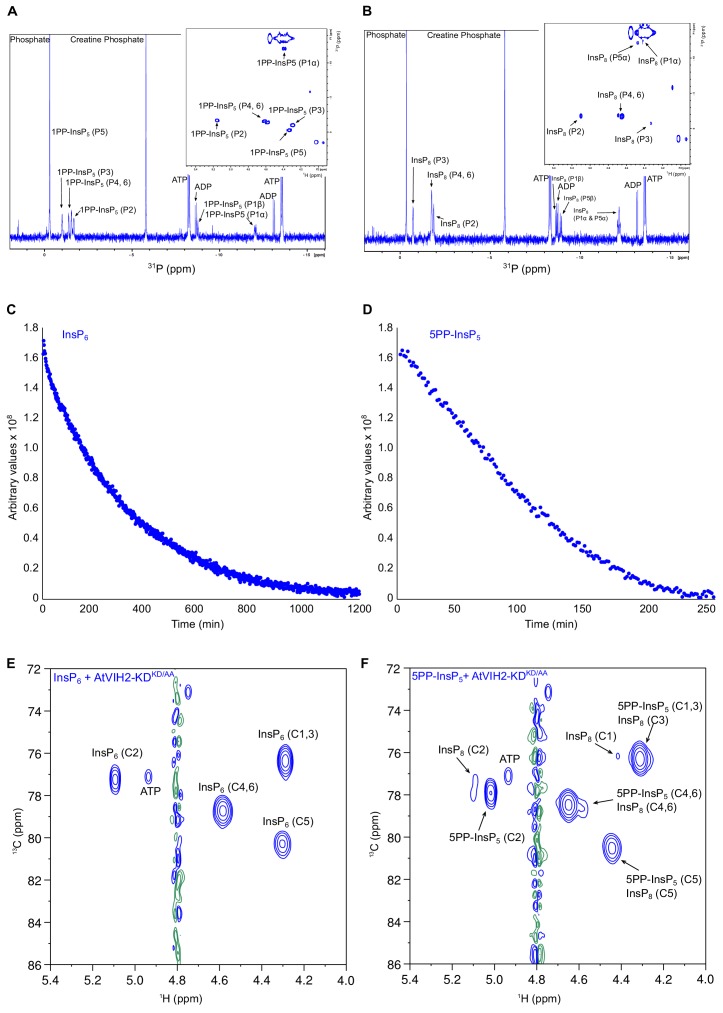
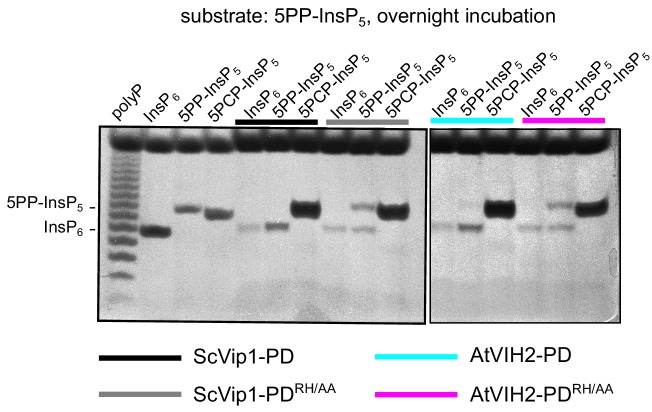
Figure 5. The AtVIH2 kinase domain has 1-kinase activity and produces 1PP-InsP5 and InsP8, the phosphatase domain is a 1 - and 5 - pyrophosphatase.
(A, B) 2D 1H-13C-HMBC spectra of the products produced by plant AtVIH2-KD (blue trace) and human HsPPIP5K2-KD (red trace) in the presence of InsP6 (A) or 5PP-InsP5 (B). Substrate standards are colored in yellow. (C) Decay of the InsP6 or 5-PP-InsP5 substrate during the NMR-time course experiment shown in (A) and (B), respectively. A fit of the initial decay indicates a turnover number of ~0.4/min with InsP6 as a substrate and ~1/min using 5-PP-InsP5 as a substrate. (D) Qualitative native PAGE phosphatase activity assay. Reactions containing recombinant phosphatase domain of AtVIH2 (AtVIH2-PD,~5 µg) or ScVip1 (ScVip1-PD,~27 µg) were incubated with 175 μM of either InsP6 (black), 1PP-InsP5 (blue), 5PP-InsP5 (gray) or a non-hydrolyzable 5PCP-InsP5 analog (orange) for 16 hr at 37°C. 40 μl of the reaction were separated in a 35% acrylamide gel. The bands corresponding to InsP6 and 1 or 1/5PP-InsP5 are indicated by an arrowhead. (E) Malachite green-based phosphatase activity assay. Reactions containing recombinant AtVIH2-PD (~5 µg) or ScVip1-PD (~27 µg) were incubated with 175 μM InsP6, 1PP-InsP5, 5PP-InsP5 or 5PCP-InsP5 for 16 hr at 37°C (left). 1 mM Mg2+ or 5 mM EDTA were supplemented as indicated (right; 5PP-InsP5 only). Recombinant HsPPIP5K2-KD (~17 µg) was used as a negative control and tested only with 5PP-InsP5 (right). Reactions were performed in quadruplicates and released orthophosphate was quantified using a malachite green assay (Baykov et al., 1988). (F) NMR time course experiment comparing the phosphatase activities of 2 µM ScVip1-PD and ScVip1-PDRH/AA using 40 µM [13C6]5PP-InsP5 as substrate. Samples were measured in a pseudo-2D spin-echo difference experiment and the relative intensities of the C2 peaks of InsP6 and 5PP-InsP5 were quantified.