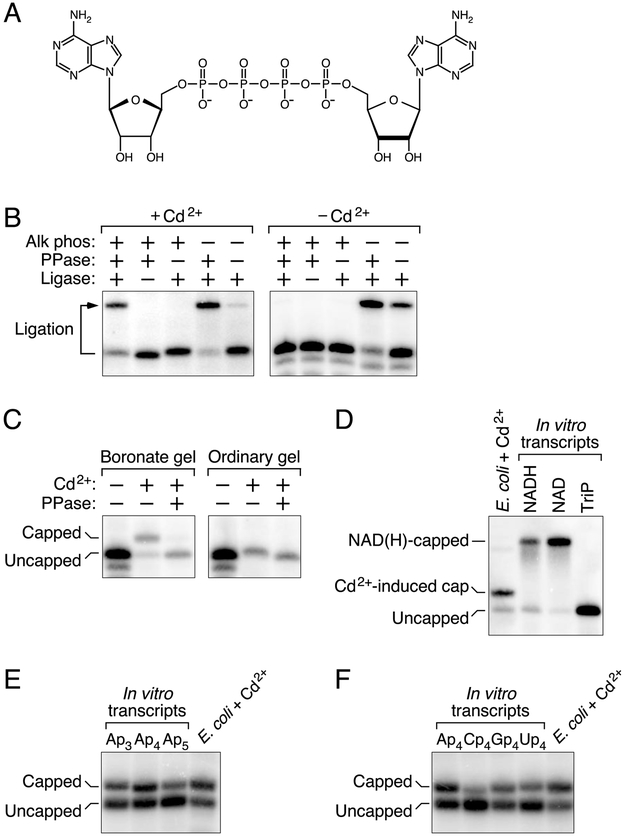
Figure 1. Detection of capped RNA in E. coli cells treated with cadmium chloride.
(A) Structure of the dinucleoside tetraphosphate AP4A.
(B) Protection of the 5′ phosphates of yeiP mRNA. Total RNA from E. coli cells that had or had not been treated with cadmium chloride was treated sequentially with alkaline phosphatase (Alk phos) and tobacco acid pyrophosphatase (PPase), and any monophosphorylated yeiP transcripts that resulted were detected by the ability of their 5′ termini to undergo splinted ligation to a DNA oligonucleotide (Ligase). The bent arrow leads from the full-length yeiP transcript to its ligation product. RNAs migrating more rapidly than the full-length transcript correspond to degradation intermediates of yeiP mRNA in E. coli; these would not have undergone splinted ligation regardless of their 5′ phosphorylation state. See also Fig. S1A.
(C) Detection of capped yeiP mRNA by boronate gel electrophoresis. Total RNA from E. coli cells that had or had not been treated with cadmium chloride was cleaved site-specifically 69 nt from the yeiP 5′ end with a 10-23 deoxyribozyme (Santoro and Joyce, 1997) so as to generate a 5′-terminal fragment bearing a 2′,3′ cyclic phosphate rather than a vicinal diol at its 3′ end. The RNA was then subjected to electrophoresis on a boronate gel or an ordinary polyacrylamide gel, with or without prior decapping (PPase), and yeiP RNA was detected by Northern blotting (Luciano and Belasco, 2019).
(D) Distinct electrophoretic mobility on a boronate gel of capped yeiP mRNA from cadmium-treated cells and NAD- or NADH-capped yeiP standards synthesized in vitro. TriP, triphosphorylated yeiP standard.
(E) Similar electrophoretic mobility on a boronate gel of capped yeiP mRNA from cadmium-treated cells and Ap3-, Ap4-, and Ap5-capped yeiP standards synthesized in vitro.
(F) Similar electrophoretic mobility on a boronate gel of capped yeiP mRNA from cadmium-treated cells and Ap4-, Cp4-, Gp4-, and Up4-capped yeiP standards synthesized in vitro.