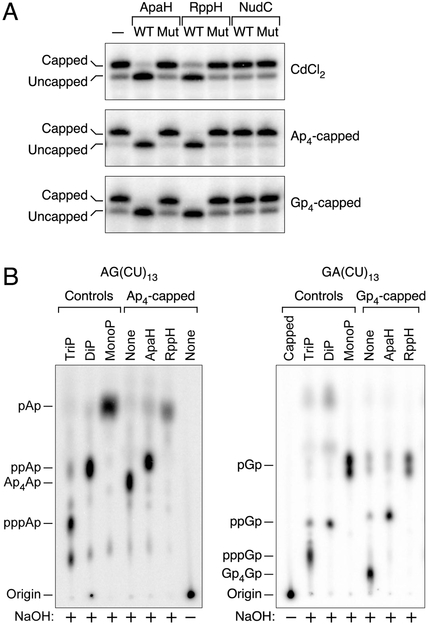
Figure 2. Decapping enzymes and their reaction products.
(A) Decapping in vitro. Purified ApaH, RppH, and NudC and catalytically inactive mutants thereof were each tested for their ability to decap yeiP mRNA that had been extracted from cadmium-treated cells (CdCl2) or had been synthesized with a 5′-terminal Ap4 or Gp4 cap by in vitro transcription. Decapping was detected by boronate gel electrophoresis and blotting. WT, wild-type enzyme; Mut, mutant enzyme lacking catalytic activity; –, no enzyme. See also Figs. S2A, S3, and S7C.
(B) Phosphorylation state of the RNA product of decapping by ApaH or RppH. Ap4-capped AG(CU)13 (left) and Gp4-capped GA(CU)13 (right) bearing a single 32P label between the first and second nucleotides were treated with purified ApaH or RppH. The radiolabeled products were subjected to alkaline hydrolysis (NaOH) and then analyzed by thin layer chromatography. Control markers were generated by hydrolyzing triphosphorylated (TriP), diphosphorylated (DiP), or monophosphorylated (MonoP) AG(CU)13 and GA(CU)13 without prior enzyme treatment.