Abstract
Background and Purpose:
Automated computed tomography perfusion (CTP) is recommended to inform selection of stroke patients for thrombectomy >6 hours from last known normal (LKN). However, artifacts on automated perfusion output may overestimate the tissue at risk leading to misclassification of thrombectomy eligibility in some patients.
Methods:
We conducted a retrospective multi-site study of consecutive patients with anterior large vessel occlusion (LVO) undergoing CTP (6/2017–12/2017). The primary outcome was the RAPID automated Tmax >6s volume that was discordant with clinical symptoms and vessel imaging, manually assessed by two independent readers. The discordant penumbral volume was compared to the automated output and corrected mismatch ratios were generated.
Results:
Of 410 consecutive patients who underwent CTP for suspected stroke, 60 (15%) had acute anterior circulation LVO. Of these, 26 (43%) had Tmax >6s abnormalities discordant with clinical symptoms and vessel imaging. There was strong interrater agreement on artifact volume (r2=0.927). Among patients with discordant Tmax imaging, the median artifactual volume was 12cc (IQR 3–21cc), accounting for a median of 8% of the automated Tmax >6s volume (IQR 3–16%, range 1–64%). Recalculation of the Tmax >6s volume resulted in 1 patient being reclassified as having an “unfavorable” mismatch ratio (2.04 to 1.40).
Conclusion:
Nearly half of patients had evidence of artifactual penumbral imaging on automated CTP, which rarely lead to misclassification of thrombectomy eligibility. While artifactual findings are reliably identified by trained raters, our results emphasize the need to evaluate CTP results with knowledge of the patient’s clinical symptoms and vascular imaging.
Keywords: CT scan, Cerebral infarction, Large vessel occlusion, Perfusion imaging, Imaging artifacts
INTRODUCTION
Perfusion-weighted imaging is critical to the selection of acute ischemic stroke patients for endovascular thrombectomy in the extended time window (6 to 24 hours after time last seen normal).1 CT perfusion (CTP) and MR perfusion can estimate brain regions with high probability of irreversible infarction (ischemic core), and areas at risk of infarction (hypoperfusion volume). The DAWN2 and DEFUSE 33 trials showed that stroke patients who present with large vessel occlusion (LVO) in the later time window and meet perfusion imaging criteria are likely to experience significant benefit from endovascular thrombectomy. While the DAWN2 trialists did not require a minimum penumbral volume (hypoperfusion volume minus ischemic core) or a minimum mismatch ratio (hypoperfusion volume divided by ischemic core) for study inclusion, DEFUSE 33 and other acute thrombectomy trials restricted enrollment based on penumbral volumes and mismatch ratios. In DEFUSE 3 and SWIFT PRIME,4 patients were required to have a penumbral volume ≥15cc and a mismatch ratio >1.8, while patients in EXTEND-IA5 required a mismatch ratio >1.2.
Automated perfusion imaging has permitted rapid and unbiased interpretation of physiologic data. According to recent AHA guidelines, the eligibility criteria for DAWN or DEFUSE 3 should be used for the determination of thrombectomy eligibility in the extended time window.1 However, like any other imaging modality, automated perfusion imaging may have artifactual abnormalities. While software platforms like RAPID (iSchemaView, Inc., Redwood City, CA) correct for some degree of motion, artifacts may persist for a variety of reasons and become incorporated in the final volumetric assessment. The clinical ramifications of these automated artifacts on potential stroke treatment decision making has not been thoroughly explored.
We hypothesized that automated perfusion imaging artifacts using CTP are common and lead to overestimation of the final hypoperfusion volume output in a subset of patients. This may ultimately lead to inappropriate determination of thrombectomy eligibility according to current AHA guidelines for some patients.1
METHODS
Patient selection
We retrospectively evaluated a registry of consecutive adult patients >18 years of age who presented with suspected acute ischemic stroke and underwent CT angiography (CTA) of the head and neck and CTP at 3 tertiary care centers in Philadelphia (06/1/2017-12/31/2017). Determination to obtain CTP was made at the discretion of the treating physician. In general, at these centers, patients who present with acute onset of neurologic dysfunction suggestive of stroke within 24h of last known normal (LKN) undergo CT/CTA/CTP, including patients transferred for consideration of thrombectomy. Patients were included in this analysis if they had symptoms and vessel imaging confirmation of an acute, unilateral anterior LVO (internal carotid artery (ICA) terminus or M1 or M2 segments of the middle cerebral artery (MCA). Patients were excluded if they had an unknown LKN or if the time LKN was >24h prior to CTP acquisition.
Imaging
At each site, CTP images were acquired following CTA of the head and neck. Iodinated contrast (100mL Isovue-370) was divided into 2 equivalent doses and administered intravenously through a 20-gauge (or larger) right antecubital catheter, separated by 2-minute intervals. CTP studies were postprocessed using RAPID software (iSchemaView, Inc., Redwood City, CA) to generate automated, motion-corrected, deconvolution-based maps of the ischemic core and penumbra, as in recently published trials.2–4,6 Relative cerebral blood flow (rCBF <30%) and time-to-maximum of the tissue residue function (Tmax >6s) were calculated, based on consensus recommendations.7
The unenhanced CT and CT angiogram of the head and neck were interpreted by a single reader (JS) with knowledge of the patient’s presenting clinical symptoms, and graded using the Alberta Stroke Program Early CT Scale (ASPECTS) score.8 Separately, the automated CTP output (rCBF <30% and Tmax >6s volumes) using RAPID were abstracted from the radiology reports.
Volumetric assessment
Two readers (AO and JPK) manually reviewed and adjudicated CTP regions of interest. Each reader was aware of the presence and location of LVO as well as the extracranial and intracranial vessel status (including vascular variants and regions of stenosis) and clinical symptoms. The reader manually calculated the true hypoperfusion volume for each patient, excluding areas of hypoperfusion that were present outside of expected vascular distributions or outside of brain tissue (e.g., CSF spaces, sinuses, skull, etc.) using standardized cerebrovascular maps9 as references. First, the reader extracted each representative Tmax >6s axial image from the RAPID output and loaded it into an opensource imaging platform (ImageJ, National Institutes of Health, Bethesda; http://imagej.nih.gov/ij). Tmax >6s regions were then manually outlined based on concordance with the clinical history and vessel imaging (See online supplement). A concordant region of hypoperfusion was defined by any Tmax >6s abnormality located within the anticipated vascular territory based on clinical symptoms and CTA findings. A discordant (or artifactual) region of hypoperfusion was defined by any Tmax >6s abnormality located beyond the anticipated territory. Artifactual regions were further subdivided based on cerebral hemisphere (ipsilateral to the LVO or contralateral) and infratentorial. The outlined regions from each axial image were summated into a final 2-dimensional area. Three-dimensional volume estimates were then calculated for each patient’s concordant and artifactual hypoperfusion regions as the sum of the total 2-dimensional estimates using ImageJ. For the analyses, we used the mean of the 2 raters’ estimates (even if only 1 rater identified a Tmax >6s artifact because it was thought that the overall artifactual volume might be small enough for 1 rater to overlook). The inter-rater agreement between volumetric measurements was assessed using Pearson’s correlation coefficient.
Hypoperfusion artifacts were attributed to excess motion if RAPID identified ≥2 degrees of patient motion in any combination of the X, Y, or Z planes during image acquisition. Given the limited available data on motion correction with RAPID, 2 degrees of motion excess was selected as the threshold for motion artifact a priori on the basis of our center’s experience using RAPID. Other artifacts were deemed cryptogenic. Hypoperfusion artifacts were also categorized by location: (1) intraventricular if they occurred within the ventricular system; (2) extra-territorial (both adjacent or remote [including the contralateral hemisphere]), if they were found in unrelated vascular territories without evidence of other vessel occlusion; and (3) dural or calvarial if there was involvement outside of the cerebrum (Figure 1).
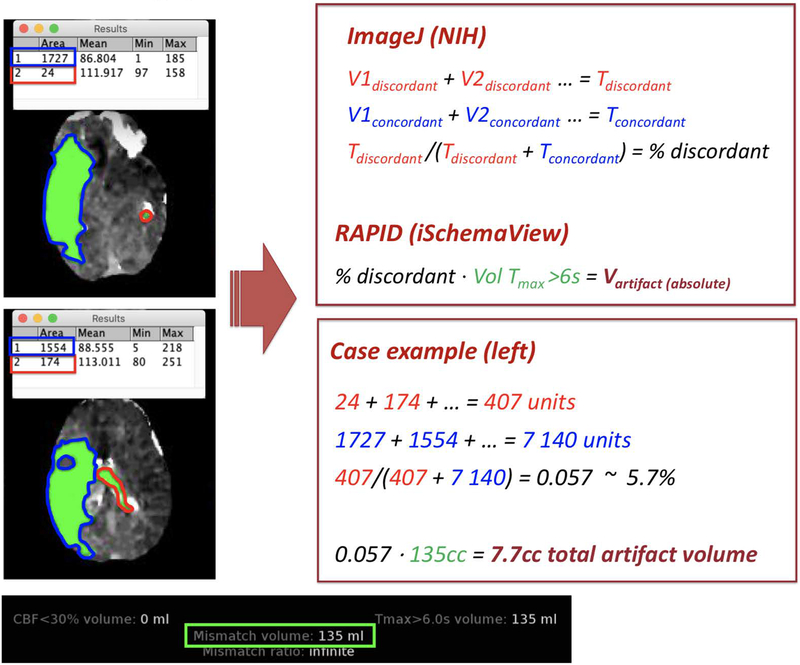
Figure 1.
Example calculations for true and artifactual regions of tissue hypoperfusion using the Tmax >6s threshold from the RAPID output. The patient presented with a right M1 occlusion and was found to have a 135cc region of hypoperfusion on RAPID with some additional regions of the intraventricular space and remote left hemisphere also being identified in the final Tmax >6s volume. True and artifactual regions were manually outlined by 2 independent readers using ImageJ (NIH), and the average volumes of true and artifactual hypoperfusion were used in the analyses. In this example, only two of the axial RAPID images are shown. The artifactual hypoperfusion region takes up a total image area of 407 units of space while the true hypoperfusion region takes up a total image area of 7,140 units of space. Converting this to cubic centimeters (or milliliters) of brain tissue, the final artifactual hypoperfusion volume was estimated at 7.7cc.
Statistical analysis
Descriptive statistics were used to describe patient characteristics. Normality of data was assessed graphically and confirmed using the Shapiro-Wilk test. Categorical data were presented as percentages, and non-normally distributed continuous data were reported as medians with interquartile range (IQR) and overall range. Unadjusted logistic regression was used to estimate the association between key demographic and clinical factors, and the presence of hypoperfusion artifact. This model was adjusted for clinical and radiographic variables significant to p<0.1 in univariable regression, and was clustered by hospital. For the adjusted logistic regression model, to avoid confounding by size of artifactual volume, only the non-artifactual Tmax >6s volume was incorporated into the final model. All tests were performed at the two-sided level using STATA 15.0 (College Station, TX), and p-values <0.05 were considered statistically significant.
This study was approved by our local Institutional Review Board with waiver of informed consent.
RESULTS
Four-hundred ten patients underwent CTP; 26 (6%) were excluded due to imaging indication other than acute stroke, 33 (8%) due to time from LKN to CTP >24 hours or unclear time LKN, 173 (42%) due to a final diagnosis other than stroke (stroke mimic or transient ischemic attack), and 118 (29%) for having no visible occlusion.
Of the remaining 60 patients who met inclusion criteria, the median age was 78 years (IQR 64–84), 36 (60%) were female, and 35 (58.3%) were non-White (Table 1). The median NIHSS was 16 (IQR 11–22). Most of the included patients (n=52) were evaluated at 1 hospital, which is the sole center for endovascular intervention between the 3 hospitals. The median time from LKN to CTP acquisition was 374min (IQR 226min – 770min), with no difference in delay from LKN to CTP among patients with hypoperfusion artifact (unadjusted OR 1.01 per hour, 95%CI 0.93–1.10, p=0.74).
Table 1.
Patient demographics.
| Overall (n=60) |
Tmax >6s without artifact (n=31) |
Tmax >6s with artifact (n=26) |
p-value | |
|---|---|---|---|---|
| Age, median years (IQR) | 78 (64–84) | 79 (68–83) | 71 (58–85) | 0.37 |
| Female, number (%) | 36 (60%) | 21 (68%) | 13 (50%) | 0.17 |
| Race, number (%) | 0.03 | |||
| Caucasian | 25 (42%) | 9 (29%) | 15 (58%) | |
| Black | 19 (31%) | 10 (32%) | 8 (31%) | |
| Other | 16 (27%) | 12 (39%) | 3 (12%) | |
| Baseline NIHSS, median (IQR) | 16 (11–22) | 15 (9–24) | 17 (15–22) | 0.41 |
| Time from LKN to CTP, median minutes (IQR) | 374 (216–770) | 360 (187–720) | 338 (216–821) | 0.96 |
| Imaging data | ||||
| Unenhanced CT ASPECTS score, median (IQR) | 8 (6–9) | 8 (6–9) | 8 (6–9) | 0.45 |
| ICA occlusion, number (%) | 20 (40%) | 11 (36%) | 8 (31%) | 0.71 |
| M1 occlusion, number (%) | 28 (47%) | 15 (48%) | 13 (50%) | 0.90 |
| M2 occlusion, number (%) | 15 (25%) | 6 (19%) | 7 (27%) | 0.50 |
| Tandem occlusions, number (%) | 3 (5%) | 3 (3%) | 2 (8%) | 0.43 |
| Hypoperfusion abnormality present, number (%) | 57 (95%) | 31 (100%) | 26 (100%) | -- |
| Hypoperfusion volume, median cc (IQR) | 104 (53–155) | 81 (14–128) | 135 (106–176) | <0.01 |
| Acute treatment | ||||
| IV tPA, number treated (%) | 17 (28%) | 7 (23%) | 9 (35%) | 0.31 |
| Thrombectomy, number treated (%) | 39 (65%) | 20 (65%) | 18 (69%) | 0.71 |
n refers to the sample number; IQR: interquartile range; NIHSS: National Institutes of Health Stroke Scale score; LKN:last known normal; CTP: computed tomography perfusion; ASPECTS: Alberta Stroke Program Early CT score; ICA: internal carotid artery; and IV tPA: intravenous tissue plasminogen activator.
Three patients with acute anterior LVO (5%) had no evidence of hypoperfusion using the automated Tmax >6s threshold (volume = 0cc). One had an occlusion of the ICA terminus whose CTP was remarkable for a 159cc penumbra using a Tmax >4s threshold, and this patient presented with an initial NIHSS of 20 and underwent thrombectomy. A second patient had an M2 occlusion with a NIHSS of 26, and a 23cc penumbra using the Tmax >4s threshold—treated with IV tPA only. A third patient had an M2 occlusion with a NIHSS of 8, and no hypoperfusion abnormality using any Tmax threshold reported by RAPID.
Hypoperfusion artifacts
The inter-rater agreement for total discordant (artifactual) hypoperfusion volume was high (r2=0.927; Table 2). When hypoperfusion artifact was identified by 1 rater and not the other, the median volumetric difference between the 2 raters was 10cc (IQR 5–22cc). Artifactual hypoperfusion volumes were similar across the 3 study sites (Table 3).
Table 2.
Inter-rater measurements of concordant and discordant hypoperfusion volumes.
| Rater 1 | Rater 2 | r2 | |||||
|---|---|---|---|---|---|---|---|
| Median hypoperfusion volume*, cc | IQR | Range | Median hypoperfusion volume*, cc | IQR | Range | ||
| All patients (n=60) | |||||||
| Total discordant volume | 0 | 0–15 | 0–262 | 0 | 0–0 | 0–250 | 0.927 |
| Ipsilateral supratentorial discordant volume | 0 | 0–4 | 0–71 | 0 | 0–0 | 0–68 | 0.533 |
| Contralateral supratentorial discordant volume | 0 | 0–4 | 0–151 | 0 | 0–0 | 0–119 | 0.870 |
| Infratentorial (discordant) volume | 0 | 0–0 | 0–103 | 0 | 0–0 | 0–115 | 0.986 |
| Patients with any discordant hypoperfusion regions (n=26) | |||||||
| Total discordant volume | 16 | 7–35 | 2–262 | 0 | 0–15 | 0–250 | 0.929 |
| Ipsilateral supratentorial discordant volume | 4 | 2–13 | 2–71 | 0 | 0–1 | 0–68 | 0.496 |
| Contralateral supratentorial discordant volume | 7 | 0–15 | 0–151 | 0 | 0–5 | 0–119 | 0.876 |
| Infratentorial (discordant) volume | 2 | 0–10 | 0–103 | 0 | 0–8 | 0–115 | 0.986 |
n refers to the sample number; IQR: interquartile range.
Hypoperfusion volume indicates the manually calculated volume of oligemic tissue identified on RAPID automated output using a Tmax> 6s threshold.
Table 3.
Artifactual hypoperfusion volumes by hospital site.
| Hospital Site 1 (n=52) |
Hospital Site 2 (n=3) |
Hospital Site 3 (n=5) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Median hypoperfusion volume, cc | IQR | Range | Median hypoperfusion volume, cc | IQR | Range | Median hypoperfusion volume, cc | IQR | Range | |
| All patients (n=60) | |||||||||
| Total discordant volume | 0 | 0–8 | 0–262 | 0 | 0–18 | 0–18 | 0 | 0–14 | 0–25 |
| Ipsilateral supratentorial discordant volume | 0 | 0–2 | 0–71 | 0 | 0–4 | 0–4 | 0 | 0–0 | 0–4 |
| Contralateral supratentorial discordant volume | 0 | 0–1 | 0–151 | 0 | 0–14 | 0–14 | 0 | 0–6 | 0–8 |
| Infratentorial (discordant) volume | 0 | 0–0 | 0–115 | 0 | 0–0 | 0–0 | 0 | 0–4 | 0–17 |
n refers to sample number; IQR: interquartile range.
Site 1 used Definition Edge (Siemens) or Definition FLASH (Siemens) CT scanners. CTP images were acquired using a 4-D spiral protocol at 70 kVp and 200 mAs.
Site 2 used Revolution HD (General Electric) or Revolution (General Electric) CT scanners. CTP images were acquired with an 80-mm wide coverage at 80 kVP and 125 mAs.
Site 3 used a Definition FLASH (Siemens) CT scanner with a similar image acquisition protocol as Site 1.
Twenty-six patients (43%) had hypoperfusion abnormalities that were discordant with the clinical symptoms and/or vascular territory, and were deemed artifactual. Four out of the 26 underwent brain MRI with diffusion-weighted imaging within 48 hours of CTP, and none had evidence of ischemia in the regions identified as artifactual. Of the 26 patients with perfusion artifact, the median artifactual volume was 12cc (IQR 3–21cc), accounting for a median per-patient proportion of 8% of the automated volume (IQR 3–16%, range 1–64%). Following manual recalculation of the mismatch ratio, 1 patient was reclassified as being ineligible for thrombectomy with a ratio that changed from 2.04 to 1.40. This patient was not recommended for thrombectomy because the hypoperfusion volume was interpreted by the treating physician as artifactual.
In fully adjusted logistic regression, clustering by hospital, artifactual Tmax >6s regions were found more commonly among Caucasian patients (OR 2.75, 95%CI 1.47–5.13, p<0.01) and patients with larger non-artifactual hypoperfusion volumes (OR 1.01 per cc, 95%CI 1.00–1.02, p<0.01).
Sixteen of the 26 cases of artifactual findings (62%) were attributed to excess patient motion, while the remaining 10 causes of artifact were cryptogenic. Among all hypoperfusion artifacts, there was involvement of adjacent vascular territories (e.g., anterior cerebral artery hypoperfusion with a middle cerebral artery occlusion) in 13 patients (50%), 13 (50%) had involvement of remote arterial territories (e.g., contralateral anterior circulation hypoperfusion), 9 (35%) had intraventricular involvement, and 4 (15%) had dural or calvarial involvement. Dural/calvarial involvement was only observed in patients with excess motion. Otherwise, there was a similar distribution of artifactual locations regardless of patient motion and no discernible pattern could be recognized (Table 4).
Table 4.
Location of artifactual hypoperfusion abnormality based on presence of excess motion.
| Motion <2 degrees (n=10) |
Motion ≥2 degrees (n=16) |
|
|---|---|---|
| Adjacent territory | 5 (50%) | 13 (50%) |
| Remote territory | 4 (40%) | 9 (56%) |
| Intraventricular | 4 (40%) | 5 (31%) |
| Dural/calvarial | 0 (0%) | 4 (25%) |
n refers to sample number. P-values not calculated for these comparisons based on small sample size and likelihood of type 1 error.
DISCUSSION
In this retrospective observational cohort of patients with anterior LVO, 43% of patients had at least some artifactual hypoperfusion abnormality on RAPID automated output. For most patients, the volume of tissue attributed to these artifacts was low, with 75% of patients having less than 15cc of artifact. In a small proportion of patients, these artifacts meaningfully overestimated the volume of salvageable brain tissue and in a rare instance, led to misclassification of thrombectomy eligibility. While we are reassured by how infrequent artifactual hypoperfusion errors occur and how small the erroneous volumes may be, we would caution clinicians to review the CTP images directly, with knowledge of typical vascular distributions, the patient’s clinical syndrome, and the CTA results. Identifying these artifactual findings may avoid inappropriate management of stroke patients, including acute interventions and unnecessary transfer for consideration of intervention, on the basis of automated imaging output.
Given the lack of available evidence of motion artifacts with automated CTP, we arbitrarily pre-selected 2 degrees of motion in any plane as the threshold for motion artifact. Interestingly, motion beyond this threshold was observed in 62% of our patients with artifact. This appears to be a moderately sensitive but highly specific threshold for identifying artifact given that we found no artifactual hypoperfusion among patients who had less than 2 degrees of any movement. However, this motion correction function may be specific to the RAPID software, and a 2-degree threshold may not be applicable to other platforms. Furthermore, this study was not intended to identify the optimal threshold for motion correction using RAPID, but it is worth exploring further. That said, our findings corroborate the results from one prior multicenter observational cohort which demonstrated that—although rare—perfusion imaging errors are most commonly due to motion artifact.10 While RAPID effectively identifies patient motion as a part of its output, and it corrects for small degrees of motion, it cannot compensate for substantial patient movement. It is important for clinicians to identify which threshold of patient motion is sufficient to contribute to artifactual perfusion abnormalities in order to avoid misinterpretation of automated imaging output.
We also observed that the size of non-artifactual hypoperfusion region independently correlated with the presence of artifact. This finding may suggest that some of these findings reflect true pathophysiology in the adjacent territory due to variability in the typical vascular boundary. Alternatively, it could be related to a “steal” phenomenon whereby collateral vessels dilate and siphon oxygenated blood from adjacent tissue—thereby creating a state of relative hypoperfusion. Some regions may also have been affected by a multifocal embolic event or diaschisis, as has been reported in posterior circulation ischemia.11 Unfortunately, only 17 patients in our cohort (28%) underwent magnetic resonance imaging after CTP, while only 4 of the patients with perfusion artifacts received MRI. Among those patients who underwent thrombectomy, MRI was acquired in all cases. Despite an increased risk of acute infarcts in additional territories due to the endovascular treatment,12 there was no evidence of ischemia in regions identified as artifactual on CTP. Interestingly, Caucasian race independently correlated with hypoperfusion artifact. To our knowledge, there is no biologic explanation to substantiate this finding, therefore we believe it to be a spurious Type I error or related to unmeasured confounders.
When evaluating the location of artifactual hypoperfusion, the most common locations were adjacent and remote vascular cerebral territories. These artifacts may reflect truly hypoperfused tissue, perhaps related to more proximal atherosclerosis, or non-atherosclerotic causes of vessel asymmetry (e.g., circle of Willis variations such as hypoplastic posterior communicating artery).13 These regions may also reflect variations in collateral anatomy across patients. However, our readers were instructed to identify regions on the CTP map that would be unequivocally supplied by an unrelated and patent intracranial artery (for example, the MCA territory hypoperfusion in the case of a right M1 occlusion as shown in Figure 2). Therefore we believe hypoperfusion lesions in adjacent but unique vascular territories were most likely to be true artifact.
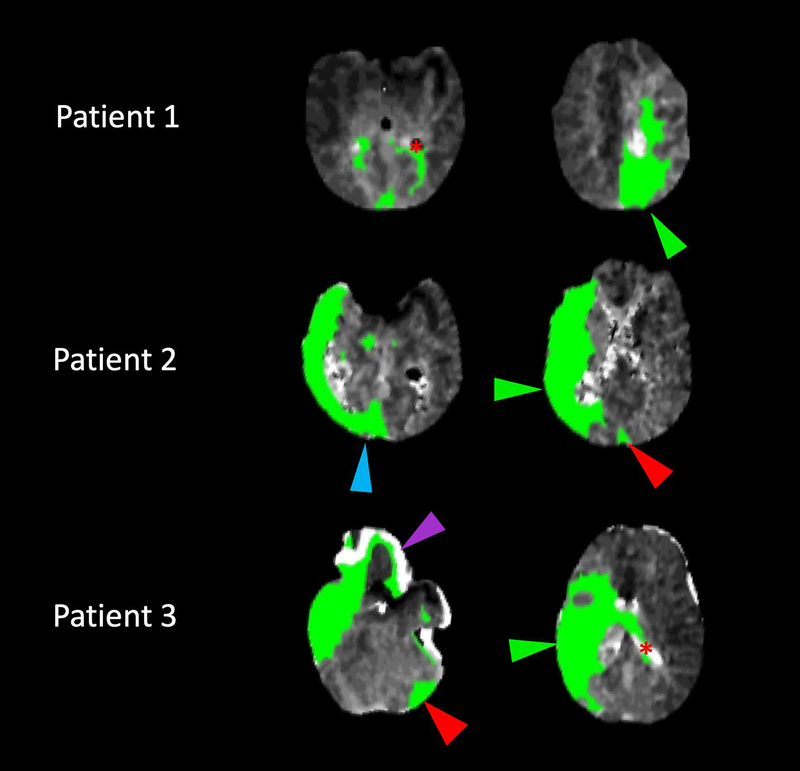
Figure 2.
Examples of artifactual CT perfusion abnormalities. Selected true and artifactual regions of hypoperfusion have been identified as follows: Green arrowheads indicate hypoperfusion regions consistent with vascular imaging and clinical status, red arrowheads indicate remote territories of hypoperfusion artifact, blue arrowheads indicate adjacent territorial artifacts, purple arrowheads indicate dural/calvarial artifacts, and red asterisks indicate intraventricular artifacts.
Patient 1: An 82-year-old woman with a recent myocardial infarction status/post coronary bypass graft with preserved ejection fraction who developed sudden weakness of her right arm and leg and aphasia, with a left anterior cerebral artery (A2) occlusion.
Patient 2: An 87-year-old gentleman with atrial fibrillation presented with left hemiparesis and neglect, with a right middle cerebral artery (M1) occlusion. His posterior circulation vessels were widely patent and the left ventricular ejection fraction normal.
Patient 3: A 64-year-old gentleman with known 80% cervical right internal carotid artery stenosis developed a new left hemiplegia and right middle cerebral artery (M1) occlusion. MRI 24 hours later confirmed no evidence of infarcts beyond this territory in spite of successful endovascular recanalization.
It is also possible that these hypoperfused regions may be truly artifactual. Some of the artifact may be the result of an imprecise perfusion map overlay due to head tilt. Tilt can lead to the false appearance of total ipsilateral hypoperfusion as the contrast material must now travel further on the affected side versus the contralateral side.13 Patient movement may also lead to misalignment of the perfusion map over the segmented axial brain images, causing adjacent but disparate vascular territories to “appear” hypoperfused. However, we found that adjacent vascular territorial perfusion artifact was not more common among patients with excess motion than among patients without motion. Only dural or calvarial involvement appeared to be specific to motion excess. Therefore, while most artifacts can be appropriately attributed to excess motion, some artifactual findings remain cryptogenic and warrant further exploration.
In addition to these observations, we found that 1 in 20 patients with acute anterior LVO had normal hypoperfusion volumes using a Tmax >6s threshold on RAPID. In 2 cases, the penumbra was captured using a 4-second threshold Tmax. While our study was not intended to assess outcomes following intervention based on perfusion imaging and LVO characteristics, 2 of the 3 patients with LVO and normal hypoperfusion imaging were treated with either IV tPA or thrombectomy. Together, these findings highlight the importance of visually inspecting the perfusion imaging and correlating regions of interest with the anticipated vascular territories based on clinical assessment or vessel status.
Our study was limited by its small sample size, retrospective nature, and predominant inclusion of patients from a single hospital. We also included patients who presented within 6 hours of LKN although these patients do not require perfusion imaging to determine candidacy for thrombectomy according to current guidelines.1 However, we found that the delay from LKN to CTP acquisition was not associated with artifactual findings. At our center, we exclusively utilized the RAPID software platform which automatically selects the AIF and VOF, which are not modifiable by the end user. Importantly, our data may not apply to all perfusion software packages. We were unable to use these data to assess the other automated perfusion processing software and there are limited head-to-head data comparing their respective performances.14 It remains unclear if the artifacts we observed in our cohort using RAPID would be similar to artifacts detected by other automated platforms such as Olea Sphere (Olea Medical) or the artificial intelligence platform Viz.ai (Viz.ai, Inc).
Ultimately, this study supports the need for experienced and knowledgeable clinicians to interpret automated perfusion imaging outputs in the context of the patient’s clinical assessment, unenhanced imaging, and vessel status. The strong correlation between raters in this study supports the idea that these artifacts can be reliably identified. Fortunately, artifactual hypoperfusion findings—while common—only rarely contribute substantially to the total hypoperfusion volume. Nevertheless, in a small proportion of cases with LVO, these findings may overestimate salvageable brain tissue and inappropriately identify patients for endovascular intervention.
Acknowledgements and Disclosures:
JS was supported by a U10 grant through the NIH (StrokeNet). The authors report no competing financial interests exist. There are no disclosures.
REFERENCES
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Engl J Med 2018;378:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. New Engl J Med 2015;372:2285–95. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med 2015;372:1009–18. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med 2015;372:1019–30. [DOI] [PubMed] [Google Scholar]
- 7.Wintermark M, Albers GW, Broderick JP, et al. Acute stroke imaging research roadmap ii. Stroke 2013;44:2628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Aspects study group. Alberta stroke programme early ct score. Lancet 2000;355:1670–4. [DOI] [PubMed] [Google Scholar]
- 9.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: Cerebral hemispheres. Neurology 1998;50:1699–708. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BC, Yassi N, Ma H, et al. Imaging selection in ischemic stroke: Feasibility of automated ct-perfusion analysis. Int J Stroke 2015;10:51–4. [DOI] [PubMed] [Google Scholar]
- 11.Carrera E, Tononi G. Diaschisis: Past, present, future. Brain 2014;137:2408–22. [DOI] [PubMed] [Google Scholar]
- 12.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 13.Best AC, Acosta NR, Fraser JE, et al. Recognizing false ischemic penumbras in ct brain perfusion studies. Radiographics 2012;32:1179–96. [DOI] [PubMed] [Google Scholar]
- 14.Austein F, Riedel C, Kerby T, et al. Comparison of perfusion ct software to predict the final infarct volume after thrombectomy. Stroke 2016;47:2311–7. [DOI] [PubMed] [Google Scholar]