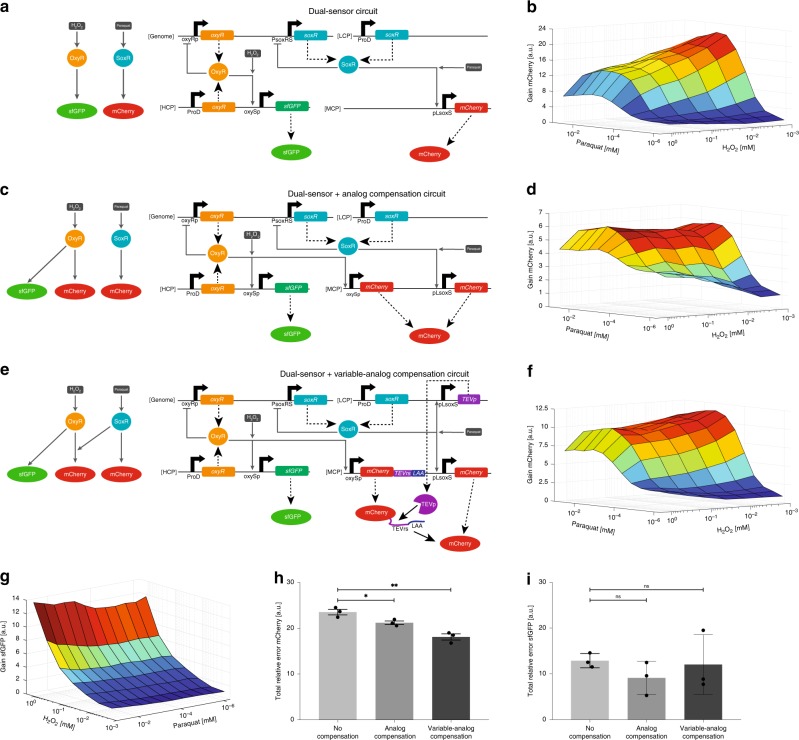
Fig. 3.
Synthetic network-level crosstalk compensation. a The first iteration of the dual-sensor circuit. SoxR is constitutively expressed from a LCP and activates mCherry expression from pLsoxS on a MCP. OxyR is constitutively expressed from a HCP and activates sfGFP expression from oxySp on the same HCP. Genomic soxR and oxyR expression are both negatively auto-regulated. Dashed large arrows represent protein production and solid small gray arrows represent transcriptional regulation. b The mCherry output fold-change relative to the minimum fluorescence for the first iteration dual-sensor strain at different concentrations of H2O2 and paraquat. mCherry expression is mostly dependent upon paraquat concentration, but there is considerable crosstalk between H2O2 and the mCherry output. c The dual-sensor circuit with analog compensation. We added an extra copy of mCherry controlled by oxySp to the dual-sensor circuit (Fig. 3a) so that the total mCherry level is controlled by both oxySp and pLsoxS promoter activity. d The mCherry output from the dual-sensor circuit with analog compensation. The crosstalk-compensation circuit reduced H2O2 crosstalk at high paraquat levels, but increased crosstalk at low paraquat levels. e The dual-sensor circuit with variable-analog compensation. To the first iteration of the dual-sensor circuit (Fig. 3a), we added an oxySp promoter on a MCP to express mCherry fused to TEVrs and an LAA degradation tag, as well as the TEVp gene on a LCP controlled by pLsoxS. TEVp post-translationally cleaves the LAA degradation sequence from the oxySp-expressed mCherry protein at the TEVrs site, stabilizing the mCherry protein. Solid black arrows represent post-translational events. f The mCherry output from the dual-sensor circuit with variable-analog compensation. H2O2 crosstalk was substantially reduced at high paraquat concentrations compared to the original dual-sensor circuit without considerably increasing crosstalk at low paraquat concentrations. g The sfGFP output fold-change relative to the minimum fluorescence for the first iteration of the dual-sensor strain (Fig. 3a). sfGFP expression is dependent upon H2O2 concentration and there is little crosstalk with paraquat. h, i The total relative mCherry (h) and sfGFP (i) error. The analog and variable-analog compensation circuits both reduce mCherry crosstalk significantly according to an unpaired two-sided t-test, without affecting the sfGFP error. The mean is shown for the total relative error calculated for each experimental replicate individually (represented by the dots). The data in Figs. 3b, d, f and g and the errors (s.e.m.) in Figs. 3h and i are derived from three biological replicates and flow cytometry experiments, each involving 30,000 events. *p < 0.05, **p < 0.005, ns indicates p > .05. In Figs. 3b, d, f, and g, the lowest concentrations of paraquat and H2O2 tested were zero, but are plotted as non-zero numbers so as to be shown on the logarithmic axes. Source data are provided as a Source Data file