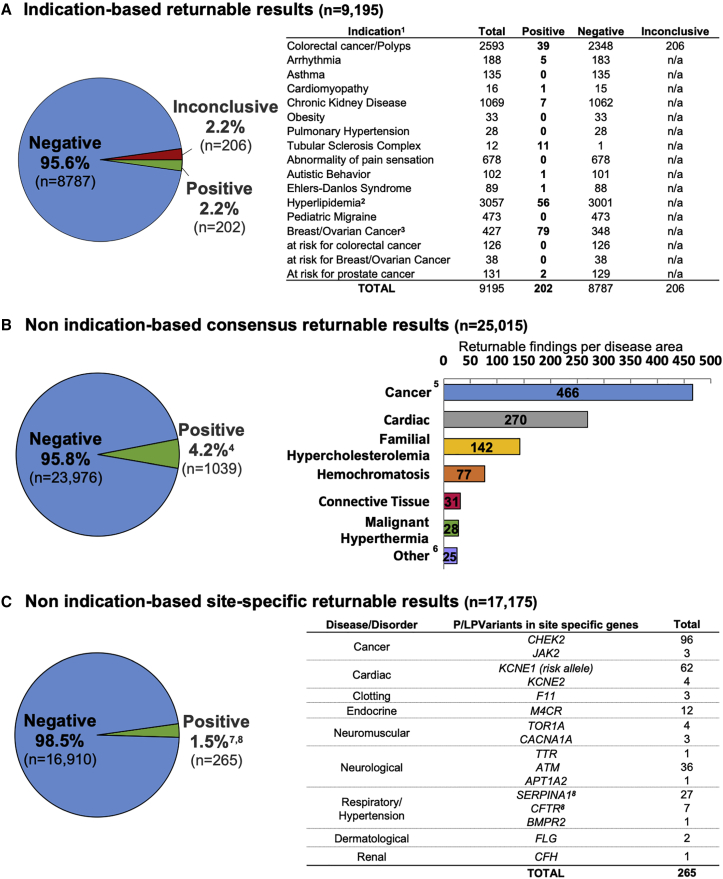
Figure 6.
Aggregate Findings Returned to Sites
The positive rate for each category of returnable findings for all 25,015 participants from the eMERGE III study is shown.
(A) Indication-based returnable results. For those with an indication for testing, the different indications are depicted. 1Four positive and two inconclusive reports had an additional secondary finding; 2587 patients had colorectal cancer and hyperlipidemia;3findings from 67 consensus genes except for 2 in CHEK2.
(B) Non indication-based consensus returnable results. Secondary findings from the consensus gene list across the entire eMERGE III cohort are broken down per disease area. 414 reports had two pathogenic variants. Skewed positive rate due to one site with sample selection based on suspicious genotype (11% positive); 5colorectal cancer (40%), breast/ovarian cancer (37%), other cancers (22%); 6other: includes immunological/inflammatory disorders, inborn errors of metabolism, endocrine disorders, neurological disorders, clotting disorders, Myhre syndrome, and neuromuscular diseases.
(C) Non indication-based site-specific returnable results. For a subset of participants, the number of pathogenic and likely pathogenic variants in site-specific additional genes that are not on the consensus list are shown. 7Ten participants had a site-specific variant and an additional consensus returnable variant. Of these ten site-specific variants returned, three were relevant to the indication for testing and seven were non-indication-based findings; 814 SERPINA1 and 5 CFTR variants were reported as carrier status.