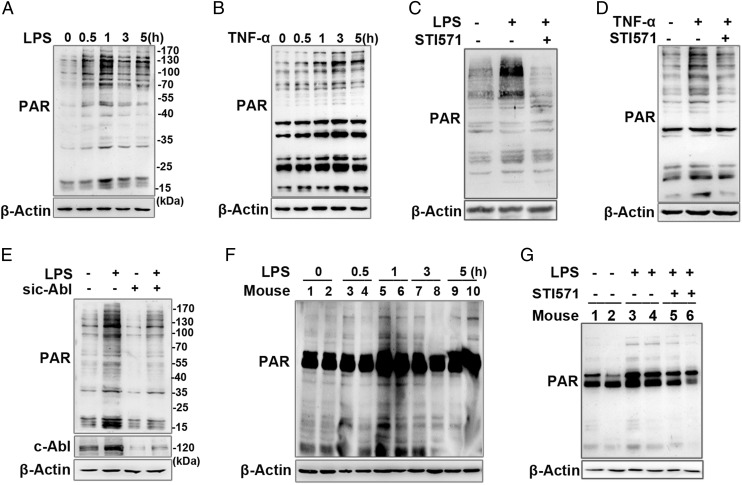
FIGURE 1.
Inflammatory agent-stimulated protein PARylation is regulated by c-Abl. (A and B) Inflammatory agents’ (LPS or TNF-α) stimulation promotes protein PARylation. RAW 264.7 cells were challenged with LPS or TNF-α for various lengths of time. Western blotting was performed to detect protein PARylation levels in whole-cell lysates. (C and D) c-Abl activity is required for inflammatory agent-stimulated protein PARylation. RAW 264.7 cells were incubated with or without LPS for 1 h or TNF-α for 3 h in the presence or absence of c-Abl inhibitor (STI571). Cell extracts were subjected to Western blotting to detect the protein PARylation. (E) siRNA of c-Abl blocked protein PARylation. RAW 264.7 cells were transfected with siRNA targeting c-Abl or the control for 48 h and then challenged with LPS for 1 h. Western blotting was performed to detect protein PARylation levels. (F) LPS induces protein PARylation in mice lungs. Mice were challenged with LPS (20 μg per mouse) through the intranasal route for different time intervals, and then the lungs were excised and homogenized. Western blotting was performed to detect the PARylation levels of each protein. (G) LPS-induced PARP1 activation in mice lung is dependent on c-Abl. Mice were challenged with LPS for 1 h with or without i.p. pretreatment of STI571(30 mg·kg−1). Lung homogenates were prepared, and Western blotting was performed to detect protein PARylation levels. Similar results were obtained from at least three independent experiments.