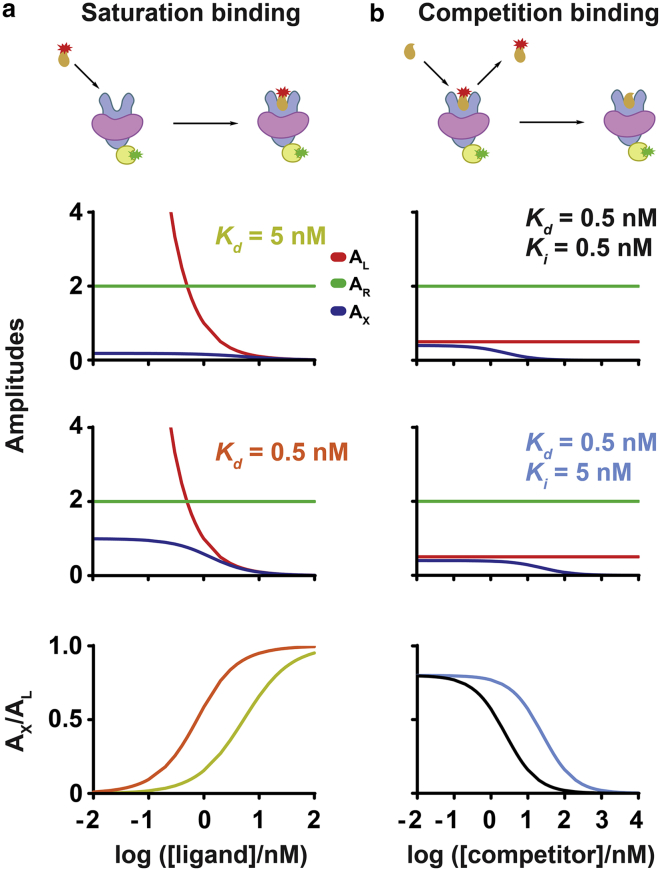
Figure 3.
Modeling binding parameters from FCCS measurements. Fractional receptor occupancy can be derived from correlation amplitudes to derive (a) saturation- and (b) competition-binding isotherms. Cartoons show the binding interactions analyzed by FCCS. Fluorescent ligand (orange with red star) recognizes a lipid-embedded (violet) membrane receptor (blue). The receptor is fused to a functional tag (yellow) labeled with a fluorophore (green). The amplitudes are modeled as a function of titrating ligand concentration for receptor, ligand, and receptor-ligand complex. For saturation binding (a), correlation amplitude plots are shown for receptor (green, AR), ligand (red, AL), and complex (blue, AX) as a function of fluorescent ligand concentrations at which ligand dissociation constants were set to 5 nM (yellow) or 0.5 nM (orange). Ligand concentration varied from 0.01 to 100 nM, and receptor concentration was kept constant at 0.5 nM. For competition-binding analysis (b), competitor concentration varied from 0.01 to 10,000 nM, and ligand and receptor concentrations were kept constant at 2 and 0.5 nM, respectively. Competition-binding isotherms can be derived by plotting AX/AL as a function of competitor concentration. Two different competition-binding cases were simulated in which ligand and receptor concentrations were kept constant while an unlabeled competitor concentration was titrated (b). The ligand and competitor affinities were assumed to be Kd = 0.5 nM, Ki = 0.5 nM (black) or Kd = 0.5 nM, Ki = 5 nM (cyan). AR and AL remain constant while AX decreases with increasing competitor concentration.