Abstract
Facial width-to-height ratio (fWHR) is associated with social dominance in human and non-human primates, which may reflect the effects of testosterone on facial morphology and behaviour. Given that testosterone facilitates status-seeking motivation, the association between fWHR and behaviour should be contingent on the relative costs and benefits of particular dominance strategies across species and socioecological contexts. We tested this hypothesis in bonobos (Pan paniscus), who exhibit female dominance and rely on both affiliation and aggression to achieve status. We measured fWHR from facial photographs, affiliative dominance with Assertiveness personality scores and agonistic dominance with behavioural data. Consistent with our hypothesis, agonistic and affiliative dominance predicted fWHR in both sexes independent of age and body weight, supporting the role of status-seeking motivation in producing the link between fWHR and socioecologically relevant dominance behaviour across primates.
Keywords: fWHR, dominance, motivation, personality, bonobo, socioecology
1. Introduction
Androgens play a key role in sexual differentiation and the promotion of competitive social behaviour across vertebrates [1]. While short-term changes in testosterone can activate the expression of dominance behaviour, androgen exposure during key developmental windows can also induce long-term organizational effects on the adult phenotype [2]. These organizational androgen effects (OAE) can cause consistent individual differences in behaviour (i.e. personality; e.g. [3]) and other androgen-sensitive phenotypes such as facial morphology [4] and digit ratios [5]. Much effort has been put into validating potential biomarkers of OAE, which may proxy prenatal and/or pubertal androgen exposure [6] and act as sexually selected signals in intra-sexual competition and mate choice [7]. The facial width-to-height ratio (fWHR), which compares the bizygomatic breadth to the distance between the brow ridge and upper lip (figure 1), has been proposed as one such OAE biomarker [8]. fWHR predicts social dominance behaviour across multiple contexts ([9–11]; but see [12]) and species (Macaca spp. [13]; Sapajus spp. [14,15]), exhibits a small degree of male-biased sexual dimorphism [9,16] and associates with other proposed OAE biomarkers such as the second-to-fourth digit ratio (2D : 4D; [17]).
Figure 1.
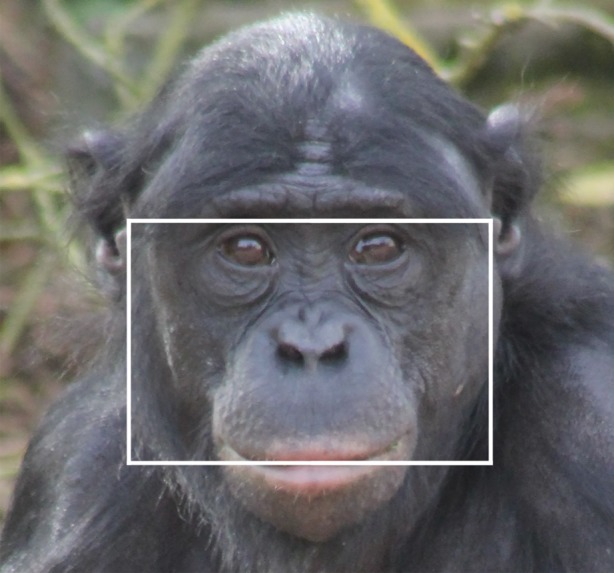
Facial width-to-height ratio. fWHR was measured by dividing the bizygomatic breadth (white box width) by the distance between the brow ridge and upper lip (white box height). The tragus was used for reference when facial hair covered the maximal cheek prominence.
While it has long been recognized that testosterone facilitates agonistic behaviour, recent work has shown that testosterone enhances status-seeking motivation and thus social dominance behaviour more generally [1,18]. Contingent upon the social context, dominance behaviour can manifest in diverse ways ranging from intimidation and aggression to gregariousness and prosocial leadership [19]. The association between fWHR and behaviour is therefore expected to reflect the relative costs and benefits of particular dominance strategies in the species and socioecology under investigation. Consistent with this hypothesis, higher fWHR has been found to predict aggression in relatively low-status men [20], but also prosocial leadership in high-status men [10]. Moreover, as expected given the higher costs of physical aggression for human females, fWHR in females is associated with self-reported dominance and verbal aggression but not with physical aggression [9,21].
Bonobos (Pan paniscus) provide a valuable system for further examining the relationship between fWHR and dominance behaviour in a novel socioecological context. As is typical for humans and mammals more generally, male bonobos experience greater exposure to testosterone during sexual maturation [22]. fWHR should therefore be larger in male bonobos if this trait is an OAE biomarker. In contrast with most human societies, however, female bonobos often exhibit social dominance over males [23,24]. Moreover, while both males and females use agonistic behaviour during competitive encounters, they also rely heavily on affiliation and coalitionary support to achieve social status [23,25,26].
Previous work in captive bonobos has demonstrated that individuals rated as being more dominant, confident and calm—thus exhibiting a higher degree of the personality dimension Assertiveness [27]—tend to receive more allogrooming, have a higher number of conspecifics in close proximity, get approached more often and receive less aggression [28]. In contrast with the personality dimension Assertiveness in brown capuchins (Sapajus spp.), however, which has previously been found to predict fWHR [14,15], Assertiveness in bonobos reflects affiliative dominance rather than aggressiveness or agonistic dominance. Consistent with the importance of coalitionary support for female dominance in bonobos [29], female bonobos also score higher in this dimension than males [28]. Bonobo Assertiveness therefore more closely aligns with the social assertiveness aspect of Extraversion in humans, which reflects a motivation towards achieving prestigious and affiliative forms of social dominance such as leadership [30]. If testosterone exposure influences facial morphology and status-seeking motivation in bonobos, fWHR should therefore be positively associated with both agonistic and affiliative dominance behaviour across males and females. In the present study, we integrate behavioural, morphometric and psychometric data to test this hypothesis.
2. Material and methods
(a). Subjects and measures
All data were collected from 2011 to 2014 on 38 sexually mature bonobos (15 males, 23 females; age range: 10–62 years, mean = 23.87 years, s.d. = 11.91 years) housed in five European zoos as part of a larger project on personality in bonobos [27,28].
(i). Facial width-to-height ratio
N.S. collected facial photographs taken ad libitum while the bonobos were on exhibit. She attempted to capture front-facing portraits for each individual while they were exhibiting a neutral expression with their mouth closed, and we discarded photos in which individuals covered their face and/or exhibited non-neutral facial expressions. This resulted in a total of 117 photographs, with an average of three acceptable images per individual. J.S.M. measured fWHR from these photos as the bizygomatic breadth divided by the distance between the brow ridge and upper lip (figure 1), which was subsequently standardized by the inter-pupil distance to adjust for heterogeneous scaling across photographs [31]. All measurements were made using Adobe Photoshop CC. A trained research assistant blind to our hypotheses independently measured fWHR in a randomly selected sample of 25% of our photographs, demonstrating appropriate single measurement reliability for fWHR as assessed by the intra-class correlation coefficient, ICC(3, 1) = 0.78.
(ii). Affiliative dominance
We assessed affiliative dominance using Assertiveness scores derived from the Hominoid Personality Questionnaire [27], which was administered to multiple human raters with extensive experience observing the bonobos. Individuals scoring high on Assertiveness were rated as being higher on traits such as independent, dominant, decisive and persistent, as well as lower on traits like submissive, anxious, vulnerable and fearful. The ratings of bonobo Assertiveness used in the present study exhibited appropriate inter-rater reliability and repeatability and were found to predict relevant behavioural measures of affiliative dominance [28].
(iii). Agonistic dominance
We used normalized David's scores (DS) [24] as a measure of individual differences in agonistic dominance [28]. Individuals with higher DS elicited a higher proportion of fleeing behaviour from their competitors during agonistic encounters. We mean-centred DS within each zoo to adjust for differences in the opportunity for agonistic encounters, thus facilitating comparison of relative agonistic dominance across zoos.
(b). Statistical analysis
All statistical analyses were conducted using linear regression models fit and interpreted within a Bayesian framework. Measurement error models were used to account for uncertainty in the mean fWHR measurement of each subject, and we used regularizing priors, for fixed effects and for the residual standard deviation, to penalize extreme estimates and reduce our risk of inferential errors [32]. Age and sex were included as covariates in all analyses. We estimated models both excluding and including body weight to determine whether potential associations between behaviour, sex and fWHR were independent of body size, as previous research has suggested that the relationship between fWHR and behaviour may be a by-product of the association between body size and facial morphology (e.g. [33]). Recent body weight measures were only available for a subset of our sample (N = 22). We used Bayesian imputation to estimate the unmeasured body weights for the remaining subjects, thus avoiding an appreciable loss of information as well as systematic bias in our estimates due to the incorrect assumption of data missing completely at random. In addition to our full main effects model, we also assessed whether sex interaction effects were present for affiliative or agonistic dominance. For comparison with [14], we further estimated a model including an interaction between affiliative and agonistic dominance.
Rather than relying on null hypothesis tests, we based our inferences on the information provided by standardized regression coefficients (), the median absolute deviation (MAD) as a robust estimate of dispersion, the 90% credible interval (CI) and the posterior probability of observing an effect in the direction of the median (i.e. p>0 or p<0). In addition, we report Cohen's f2 for our fixed effects, which provides a standardized measure of local effect size for multiple regression. Values of f2 ≥ 0.02, f2 ≥ 0.15 and f2 ≥ 0.35 are traditionally interpreted as small, medium and large effects, respectively [34]. Tildes are used throughout to denote posterior median estimates of these values. fWHR measures were standardized to z-scores, and all non-binary covariates were standardized to 2 × s.d. variance to facilitate comparison with the binary sex effect. See the electronic supplementary material for further details on our statistical analyses.
3. Results
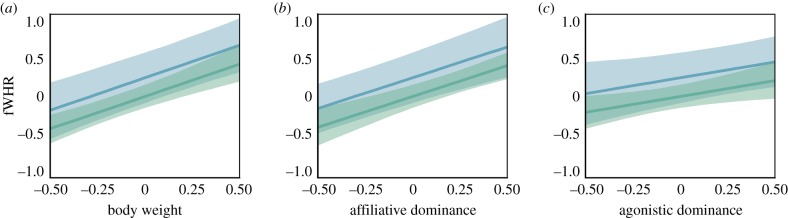
Male bonobos exhibited larger fWHR than females ( [MAD = 0.26], 90% CI [0.01, 0.89], p>0 = 0.95, ). The effect of sex was reduced, however, after controlling for body weight ( [0.22], 90% CI [−0.13, 0.62], p>0 = 0.87, ), while body weight exhibited an independent positive association with fWHR ( [0.18], 90% CI [0.59, 1.20], p>0 = 1, ; figure 2a). Controlling for body weight, age and sex, fWHR was positively associated with both affiliative (, 90% CI [0.52, 1.12], p>0 = 1, ; figure 2b) and agonistic dominance (, 90% CI [0.08, 0.77], p>0 = 0.98, ; figure 2c). There was a negative effect of age on fWHR (, 90% CI [−0.93, −0.16], p<0 = 0.99, ). Little evidence was found for sex-specific links between fWHR and affiliative (, 90% CI [−0.67, 0.64], p<0 = 0.51, ) or agonistic dominance (, 90% CI [−1.01, 0.41], p<0 = 0.75, ), and agonistic and affiliative dominance did not exhibit an interaction effect on fWHR (, 90% CI [−0.62, 0.88], p>0 = 0.61,). Overall, our main effects model accounted for a large proportion of the observed variance in fWHR ().
Figure 2.
fWHR, sex and social dominance in bonobos. Main effect model estimates (predicted median and 90% CI) for the association of male (blue) and female (green) fWHR with (a) body weight, (b) affiliative dominance and (c) agonistic dominance, conditional on average covariate values. Continuous predictors were standardized to 2 s.d. and are therefore displayed in the [−0.5, 0.5] interval for comparison with a 1 s.d. change in fWHR.
4. Discussion
We found that fWHR was associated with the expression of sociecologically relevant dominance behaviour in male and female bonobos. These findings were independent of body weight, suggesting that fWHR provides unique information about personality and dominance beyond body size (cf. [31]). While fWHR has been linked to variation in agonistic dominance styles across macaque species [13], the present study is the first to demonstrate that fWHR also predicts intraspecific variation in affiliative dominance in a non-human primate. This provides a crucial link to human research demonstrating positive associations between fWHR and prosocial behaviour in high-status contexts [10]. Indeed, the stronger association between affiliative dominance and fWHR (Cohen's ) compared to agonistic dominance () is consistent with the greater importance of coalitionary support and affiliation in bonobo societies [23,25,26], and thus, the role of testosterone for promoting socioecologically relevant status-seeking behaviour rather than aggression per se [1,18]. The observed male-bias in fWHR also provides indirect support for the role of testosterone in producing the association between fWHR and affiliative dominance, as males experience greater increases in pubertal testosterone than females [22]. Nonetheless, this sex difference was reduced after controlling for body weight, which positively predicted fWHR, suggesting that the observed sexual dimorphism in bonobo fWHR reflects allometric scaling with body size. Testosterone also influences body size [35], however, and may therefore be a common cause of this association.
We also observed a negative association between age and fWHR, consistent with previous research demonstrating declining fWHR in human populations across the lifespan [36]. Dominance ranks have been observed to vary within bonobo and chimpanzee societies across time in sex-specific ways, with critical factors such as personality, competitive ability, and social support exhibiting sex- and age-specific influences on social status (e.g. [37–39]). Given the cross-sectional nature of our study design, it remains unclear whether the observed age- and sex-independent associations between fWHR and social dominance are consistent across ontogeny. However, our data do not provide any clear support for interaction effects between sex, age and/or social dominance on fWHR (see electronic supplementary material for further analyses). These results tentatively suggest that the organizational effects of testosterone on status-seeking motivation may also stabilize associations between fWHR and observed dominance across the lifespan. Nevertheless, future longitudinal research with greater statistical power will be crucial for examining these developmental patterns.
Relatedly, the validity of fWHR as an OAE marker cannot be directly assessed without an accurate measure of androgen exposure during the critical organizational periods of brain and facial development. The hypothesized connection between fWHR and androgen exposure in humans remains unclear, as recent work has not found strong associations between fWHR and pubertal ([31]; but see [40]) or prenatal [4] testosterone levels or exposure, respectively. Moreover, fWHR is not strongly associated with polymorphisms in the androgen receptor gene, which also does not moderate the androgen–fWHR association [41]. Nonetheless, aspects of human facial width have been found to reflect prenatal testosterone [4] and exhibit male-biased sexual dimorphism across the lifespan [42]. This suggests that more complex experimental designs will be needed to disentangle the causal bases of the facial components underlying composite measures such as fWHR [43]. Investigating the mechanisms linking fWHR and behaviour is therefore an important task for future research. Given that organizational effects are hypothesized to cause the association between fWHR and behaviour, studies of baseline testosterone levels or activational effects in adults may not clarify this issue (cf. [44]).
In summary, our study demonstrates that fWHR is linked to both agonistic and affiliative forms of social dominance in a non-human species. In conjunction with previous work linking fWHR and social dominance behaviour in humans [9,10], macaques [13] and capuchins [14,15], our findings suggest that facial morphology may provide reliable cues of status-seeking motivation and testosterone exposure prior to sexual maturity. Identifying whether non-human primates use this information for social decision-making is thus a clear target for future research. For example, female rhesus macaques (Macaca mulatta) have been demonstrated to perceive differences in male facial masculinity [45], suggesting that fWHR may be a signal of intra-sexual competitive ability used in partner choice. Given that female bonobos often exhibit social dominance over males [23,24], it would be valuable to examine whether male bonobos can also perceive dominance information encoded in female faces. In addition, understanding the sociocognitive effects of facial morphology in the context of varying facial hair and coloration also remains important for determining the ecological relevance of such discriminations [46]. Irrespective of their communicative function, however, consistent associations have now been identified between fWHR, dominance rank and status-seeking motivation in humans, bonobos and capuchins. The organizational effects of testosterone may therefore be an important and phylogenetically conserved mechanism of personality across haplorhine primates.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Kevin Delijani for assistance with fWHR measurements, as well as Ginger Wickline for help in organizing this project and the Miami University Honors Program for their support. We further thank the Primate Genomics Lab and the Laboratory for Evolutionary Neuroscience at the George Washington University for helpful feedback on the project.
Ethics
The study was approved by the Scientific Advisory Board of the Royal Zoological Society of Antwerp and the University of Antwerp, Belgium, and endorsed by the European Breeding Program for bonobos. All research complied with the Association for the Study of Animal Behaviour 2012 guidelines.
Data accessibility
The dataset and R code supporting this article have been provided as electronic supplementary material.
Authors' contributions
J.S.M. measured fWHR and composed the manuscript, N.S. and J.M.G.S. gathered the photographs and agonistic dominance measurements, N.S., A.W. and J.M.G.S. collected the personality measures, J.S.M. conducted the statistical analyses with input from A.V.J. and all authors contributed to significant revision of the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This project was funded in part by the Miami University Honors Department Joanna Jackson Goldman Memorial Prize and FWO Flanders.
References
- 1.Eisenegger C, Haushofer J, Fehr E. 2011. The role of testosterone in social interaction. Trends Cogn. Sci. 15, 263–271. ( 10.1016/j.tics.2011.04.008) [DOI] [PubMed] [Google Scholar]
- 2.Schulz KM, Sisk CL. 2016. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci. Biobehav. Rev. 70, 148–158. ( 10.1016/j.neubiorev.2016.07.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GR, Kulbarsh KD, Spencer KA, Duval C. 2015. Peri-pubertal exposure to testicular hormones organizes response to novel environments and social behaviour in adult male rats. Horm. Behav. 73, 135–141. ( 10.1016/j.yhbeh.2015.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehouse AJ, et al. 2015. Prenatal testosterone exposure is related to sexually dimorphic facial morphology in adulthood. Proc. R. Soc. B 282, 20151351 ( 10.1098/rspb.2015.1351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lofeu L, Brandt R, Kohlsdorf T. 2017. Phenotypic integration mediated by hormones: associations among digit ratios, body size and testosterone during tadpole development. BMC Evol. Biol. 17, 175 ( 10.1186/s12862-017-1021-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breedlove SM. 2010. Minireview: organizational hypothesis: instances of the fingerpost. Endocrinology 151, 4116–4122. ( 10.1210/en.2010-0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puts DA. 2010. Beauty and the beast: mechanisms of sexual selection in humans. Evol. Hum. Behav. 31, 157–175. ( 10.1016/j.evolhumbehav.2010.02.005) [DOI] [Google Scholar]
- 8.Carré JM, McCormick CM. 2008. In your face: facial metrics predict aggressive behaviour in the laboratory and in varsity and professional hockey players. Proc. R. Soc. B 275, 2651–2656. ( 10.1098/rspb.2008.0873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geniole SN, Denson TF, Dixson BJ, Carré JM, McCormick CM. 2015. Evidence from meta-analyses of the facial width-to-height ratio as an evolved cue of threat. PLoS ONE 10, e0132726 ( 10.1371/journal.pone.0132726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn T, Winter NR, Anderl C, Notebaert K, Wuttke AM, Clément CC, Windmann S. 2017. Facial width-to-height ratio differs by social rank across organizations, countries, and value systems. PLoS ONE 12, e0187957 ( 10.1371/journal.pone.0187957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haselhuhn MP, Ormiston ME, Wong EM. 2015. Men's facial width-to-height ratio predicts aggression: a meta-analysis. PLoS ONE 10, e0122637 ( 10.1371/journal.pone.0122637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosinski M. 2017. Facial width-to-height ratio does not predict self-reported behavioral tendencies. Psychol. Sci. 28, 1675–1682. ( 10.1177/0956797617716929) [DOI] [PubMed] [Google Scholar]
- 13.Borgi M, Majolo B. 2016. Facial width-to-height ratio relates to dominance style in the genus Macaca. PeerJ 4, e1775 ( 10.7717/peerj.1775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefevre CE, Wilson VA, Morton FB, Brosnan SF, Paukner A, Bates TC. 2014. Facial width-to-height ratio relates to alpha status and assertive personality in capuchin monkeys. PLoS ONE 9, e93369 ( 10.1371/journal.pone.0093369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson V, Lefevre CE, Morton FB, Brosnan SF, Paukner A, Bates TC. 2014. Personality and facial morphology: links to assertiveness and neuroticism in capuchins (Sapajus [Cebus] apella). Pers. Individ. Dif. 58, 89–94. ( 10.1016/j.paid.2013.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köllner MG, Janson KT, Schultheiss OC. 2018. Commentary: Sexual dimorphism of facial width-to-height ratio in human skulls and faces: a meta-analytical approach. Front. Endocrinol. 9, 227 ( 10.3389/fendo.2018.00227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meindl K, Windhager S, Wallner B, Schaefer K. 2012. Second-to-fourth digit ratio and facial shape in boys: the lower the digit ratio, the more robust the face. Proc. R. Soc. B 279, 2457–2463. ( 10.1098/rspb.2011.2351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreher JC, Dunne S, Pazderska A, Frodl T, Nolan JJ, O'Doherty JP. 2016. Testosterone causes both prosocial and antisocial status-enhancing behaviors in human males. Proc. Natl Acad. Sci. USA 113, 11 633–11 638. ( 10.1073/pnas.1608085113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maner JK. 2017. Dominance and prestige: a tale of two hierarchies. Curr. Dir. Psychol. Sci. 26, 526–531. ( 10.1177/0963721417714323) [DOI] [Google Scholar]
- 20.Goetz SM, Shattuck KS, Miller RM, Campbell JA, Lozoya E, Weisfeld GE, Carré JM. 2013. Social status moderates the relationship between facial structure and aggression. Psychol. Sci. 24, 2329–2334. ( 10.1177/0956797613493294) [DOI] [PubMed] [Google Scholar]
- 21.Lefevre CE, Etchells PJ, Howell EC, Clark AP, Penton-Voak IS. 2014. Facial width-to-height ratio predicts self-reported dominance and aggression in males and females, but a measure of masculinity does not. Biol. Lett. 10, 20140729 ( 10.1098/rsbl.2014.0729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behringer V, Deschner T, Deimel C, Stevens JM, Hohmann G. 2014. Age-related changes in urinary testosterone levels suggest differences in puberty onset and divergent life history strategies in bonobos and chimpanzees. Horm. Behav. 66, 525–533. ( 10.1016/j.yhbeh.2014.07.011) [DOI] [PubMed] [Google Scholar]
- 23.Surbeck M, Hohmann G. 2013. Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 67, 1767–1780. ( 10.1007/s00265-013-1584-8) [DOI] [Google Scholar]
- 24.Stevens JM, Vervaecke H, De Vries H, van Elsacker L. 2007. Sex differences in the steepness of dominance hierarchies in captive bonobo groups. Int. J. Primatol. 28, 1417–1430. ( 10.1007/s10764-007-9186-9) [DOI] [Google Scholar]
- 25.Surbeck M, Deschner T, Schubert G, Weltring A, Hohmann G. 2012. Mate competition, testosterone and intersexual relationships in bonobos, Pan paniscus. Anim. Behav. 83, 659–669. ( 10.1016/j.anbehav.2011.12.010) [DOI] [Google Scholar]
- 26.Vervaecke H, De Vries H, Van Elsacker L. 2000. The pivotal role of rank in grooming and support behavior in a captive group of bonobos (Pan paniscus). Behaviour 137, 1463–1485. ( 10.1163/156853900502673) [DOI] [Google Scholar]
- 27.Weiss A, Staes N, Pereboom JJ, Inoue-Murayama M, Stevens JM, Eens M. 2015. Personality in bonobos. Psychol. Sci. 26, 1430–1439. ( 10.1177/0956797615589933) [DOI] [PubMed] [Google Scholar]
- 28.Staes N, Eens M, Weiss A, Stevens JMG. 2017. Bonobo personality: age and sex effects and links with behavior and dominance. In Bonobos: unique in mind, brain and behavior (eds Hare B, Yamamoto S). Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Tokuyama N, Furuichi T. 2016. Do friends help each other? Patterns of female coalition formation in wild bonobos at Wamba. Anim. Behav. 119, 27–35. ( 10.1016/j.anbehav.2016.06.021) [DOI] [Google Scholar]
- 30.DeYoung CG, Quilty LC, Peterson JB. 2007. Between facets and domains: 10 aspects of the Big Five. J. Pers. Soc. Psychol. 93, 880–896. ( 10.1037/0022-3514.93.5.880) [DOI] [PubMed] [Google Scholar]
- 31.Hodges-Simeon CR, Sobraske KNH, Samore T, Gurven M, Gaulin SJ. 2016. Facial width-to-height ratio (fWHR) is not associated with adolescent testosterone levels. PLoS ONE 11, e0153083 ( 10.1371/journal.pone.0153083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElreath R. 2016. Statistical rethinking: a Bayesian course with examples in R and Stan. Boca Raton, FL: CRC Press. [Google Scholar]
- 33.Kramer RS. 2015. Facial width-to-height ratio in a large sample of commonwealth games athletes. Evol. Psychol. 13, 197–209. ( 10.1177/147470491501300112) [DOI] [PubMed] [Google Scholar]
- 34.Cohen JE. 1988. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 35.Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. 2004. Endocrine control of body composition in infancy, childhood, and puberty. Endocr. Rev. 26, 114–146. ( 10.1210/er.2003-0038) [DOI] [PubMed] [Google Scholar]
- 36.Hehman E, Leitner JB, Freeman JB. 2014. The face–time continuum: lifespan changes in facial width-to-height ratio impact aging-associated perceptions. Pers. Soc. Psychol. Bull. 40, 1624–1636. ( 10.1177/0146167214552791) [DOI] [PubMed] [Google Scholar]
- 37.Altschul DM, Hopkins WD, Herrelko ES, Inoue-Murayama M, Matsuzawa T, King JE, Ross SR, Weiss A. 2018. Personality links with lifespan in chimpanzees. eLife 7, e33781 ( 10.7554/eLife.33781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furuichi T, Ihobe H. 1994. Variation in male relationships in bonobos and chimpanzees. Behaviour 130, 211–228. ( 10.1163/156853994X00532) [DOI] [Google Scholar]
- 39.Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE. 2016. Chimpanzee females queue but males compete for social status. Sci. Rep. 6, 35404 ( 10.1038/srep35404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welker KM, Bird BM, Arnocky S. 2016. Commentary: facial width-to-height ratio (fWHR) is not associated with adolescent testosterone levels. Front. Psychol. 7, 1745 ( 10.3389/fpsyg.2016.01745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenbruch AB, Lukaszewski AW, Simmons ZL, Arai S, Roney JR. 2018. Why the wide face? Androgen receptor gene polymorphism does not predict men's facial width-to-height ratio. Adapt. Hum. Behav. Physiol. 4, 138–151. ( 10.1007/s40750-017-0084-x) [DOI] [Google Scholar]
- 42.Robertson JM, Kingsley BE, Ford GC. 2017. Sexually dimorphic faciometrics in humans from early adulthood to late middle age: dynamic, declining, and differentiated. Evol. Psychol. 15, 1474704917730640 ( 10.1177/1474704917730640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixson BJ. 2018. Is male facial width-to-height ratio the target of sexual selection? Arch. Sex. Behav. 47, 827–828. ( 10.1007/s10508-018-1184-9) [DOI] [PubMed] [Google Scholar]
- 44.Bird BM, Jofré VSC, Geniole SN, Welker KM, Zilioli S, Maestripieri D, Arnocky S, Carre JM. 2016. Does the facial width-to-height ratio map onto variability in men's testosterone concentrations? Evol. Hum. Behav. 37, 392–398. ( 10.1016/j.evolhumbehav.2016.03.004) [DOI] [Google Scholar]
- 45.Rosenfield KA, Semple S, Georgiev AV, Maestripieri D, Higham JP, Dubuc C. 2019. Experimental evidence that female rhesus macaques (Macaca mulatta) perceive variation in male facial masculinity. R. Soc. open sci. 6, 181415 ( 10.1098/rsos.181415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixson BJ, Lee AJ, Sherlock JM, Talamas SN. 2017. Beneath the beard: do facial morphometrics influence the strength of judgments of men's beardedness? Evol. Hum. Behav. 38, 164–174. ( 10.1016/j.evolhumbehav.2016.08.004) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset and R code supporting this article have been provided as electronic supplementary material.