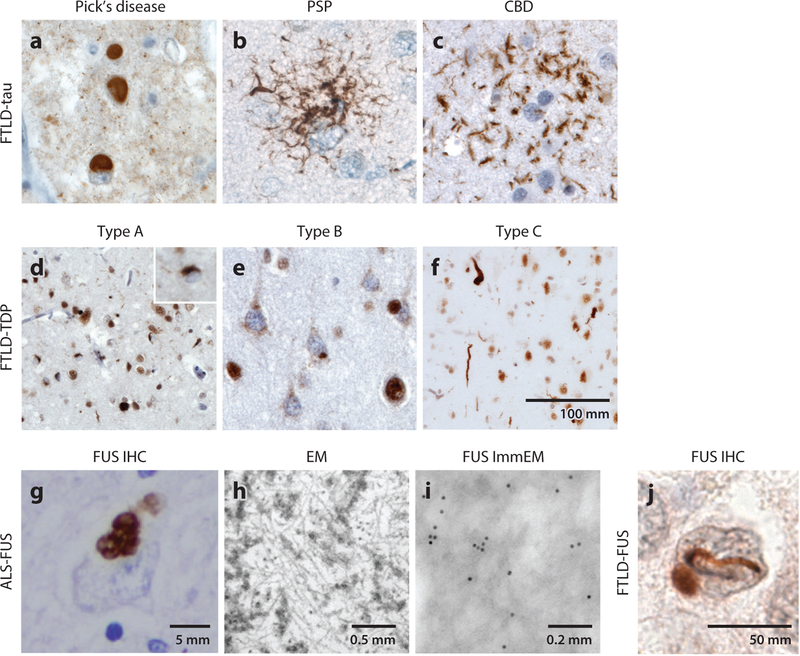
Figure 2.
Neuropathology of FTLD. Subtypes of (a–c) FTLD-tau and (d–f) FTLD-TDP are distinguished by the morphology and distribution of their characteristic lesions. (a) Pick bodies in Pick’s disease; (b) a tufted astrocyte in PSP; (c) an astrocytic plaque in CBD; (d) small compact or crescentic neuronal cytoplasmic inclusions and short, thin neuropil threads in FTLD-TDP type A; (e) diffuse granular or speckled neuronal cytoplasmic inclusions, with a relative paucity of neuropil threads, in FTLD-TDP type B; and (f) long, tortuous dystrophic neurites in FTLD-TDP type C. TDP-43 can be seen within the nucleus in neurons lacking inclusions, but it mislocalizes to the cytoplasm within inclusion-bearing neurons. (g) Immunohistochemical stain for FUS highlights the prominent FUS-positive staining in basophilic inclusions in the cytoplasm of spinal motor neurons in an ALS-FUS case. (h,i) Ultrastructural analyses of the same case as shown in panel g show that these inclusions (h) contain filamentous aggregates measuring 15–20 nm in diameter and (i) are immunoreactive for FUS antibody. (j) The most common subtype of FTLD-FUS is aFTLD-U, characterized by signature FUS immunoreactive vermiform intranuclear inclusions. Immunostains were performed using antibodies to (a) 3R tau, (b,c) phospho-tau (CP-13), (d–f) TDP-43, and (g,i,j) FUS. The scale bar in panel f is applicable to panels a–e. Abbreviations: aFTLD-U, atypical FTLD-ubiquitin; ALS, amyotrophic lateral sclerosis; CBD, corticobasal degeneration; FTLD, frontotemporal lobar degeneration; EM, electron microscopy; IHC, immunohistochemistry; ImmEM, immunogold electron microscopy; PSP, progressive supranuclear palsy.