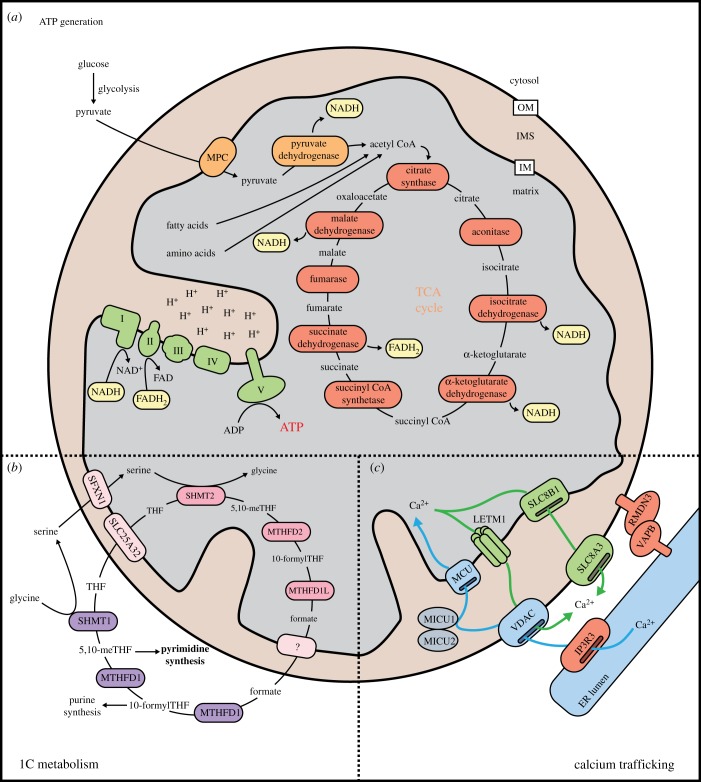
Figure 3.
Mitochondria coordinate essential metabolic processes. (a) Mitochondria are best known for housing the protein machinery required for generating ATP. When oxygen is available, most cells will generate ATP through oxidative phosphorylation, where electrons harvested through catabolic reactions are used to power ATP synthase. Electrons are obtained through the TCA cycle, which occurs in the matrix and consists of eight enzymatic reactions. Acetyl-CoA is the primary input for the TCA cycle, and can be obtained through metabolism of glucose, fatty acids and amino acids. Electrons extracted during the TCA cycle are loaded onto NAD+ and FAD2+. Electrons are subsequently transferred from NADH and FADH2 onto Complexes I and II of the electron transport chain. Electrons are passed through Complexes III and IV, which transport protons into the intermembrane space. Protons are allowed to flow back into the matrix through ATP synthase (Complex V), which uses the energy of the proton gradient to convert ADP to ATP. (b) Mitochondrial one-carbon (1C) metabolism comprises a series of parallel and reversible reactions which occur in the cytosol and mitochondrial matrix. In proliferating cells, the reaction normally proceeds in a specific direction such that formate produced within mitochondria can be used for biosynthetic processes in the cytosol. Within the mitochondria, THF and serine imported from the cytosol are acted upon sequentially by SHMT2, MTHFD2 and MTHFD1 L to produce formate, which is exported back into the cytosol. Cytosolic MTHFD1 loads formate onto THF to form charged folate intermediates that can be used to synthesize purine and pyrimidine nucleotides. Mitochondrial 1C metabolism is also an important source of glycine. (c) The mitochondrial matrix functions as an important storage site for calcium ions. Mitochondrial calcium uptake often occurs at ER contact sites, where large volumes of Ca2+ can be released through IP3R3. Calcium can pass freely through the outer membrane via VDAC channels and is transported across the intermembrane space and inner membrane through the coordinated function of a MICU1/MICU2 dimer docking to MCU in the inner membrane. Calcium can exit the mitochondrial matrix through LETM1 or SLC8B1 (in exchange for H+ or Na+, respectively) and can cross the outer membrane through VDACs or NCX3.