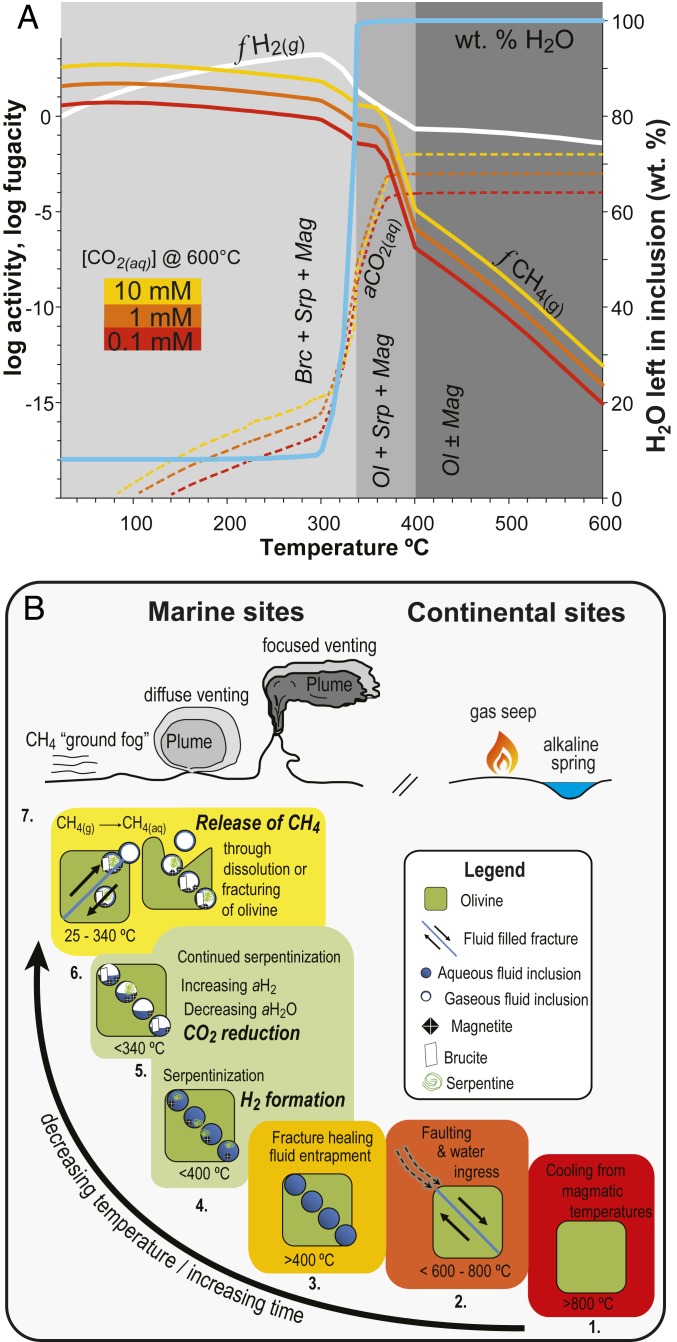
Fig. 4.
Equilibrium model (A) and conceptual model (B) of CH4 formation in olivine-hosted fluid inclusions. (A) Predicted equilibrium composition of a trapped fluid interacting with its olivine host in a closed system as a function of temperature and initial CO2(aq) concentration (0.1 to 10 mM). Also shown is the mass of reactant water as a function of temperature. Above ∼400 °C, olivine (Ol) is stable, CO2(aq) is the dominant carbon species, and virtually none of the liquid water is consumed by hydrous minerals. Below ∼340 °C, brucite (Brc), serpentine (Srp), and magnetite (Mag) form at the expense of olivine (unstable), and most of the water is consumed while abundant H2(g) and CH4(g) are formed. See Methods for modeling details. (B) Conceptual model illustrating the sequence of events that lead to the formation of CH4 in olivine-hosted inclusions and its subsequent release. Cooling from magmatic temperatures (1) to below the brittle–ductile transition temperature (2) allows water to penetrate the rock. Healing of fluid-filled fractures (3) forms secondary fluid inclusions. During cooling below 400 °C, serpentinization begins (4). During cooling below 340 °C, increased H2 generation and consumption of water create conditions conducive to CH4 formation (5). Fracturing or dissolution of the olivine host releases volatiles (6), which subsequently vent at the surface (7).