Abstract
The kidney is one of the major organs affected in preeclampsia. There is evidence suggesting a role for excessive complement activation in the pathogenesis of preeclampsia. We describe a case of preeclampsia with severe features, including HELLP syndrome (hemolysis, elevated liver enzymes, low platelets) and acute kidney injury (AKI) that developed following caesarian section. The patient required renal replacement therapy. A trial of daily plasma exchange was not effective. The patient received a single dose of eculizumab, a humanised monoclonal IgG antibody that binds to complement protein C5. One week post administration of eculizumab, there was significant improvement in haematologic, hepatic and renal function. Blood pressure had normalised and renal replacement therapy was discontinued. The use of eculizumab may have contributed to recovery of kidney function further supporting the role of complement activation in the pathogenesis of preeclampsia and associated AKI.
Keywords: renal system; obstetrics, gynaecology and fertility
Background
Preeclampsia is a pregnancy-related disorder and is a major cause of maternal and neonatal morbidity and mortality.1 In addition, preeclampsia is the most common reason for intensive care unit (ICU) admission among the obstetric emergencies.2 Preeclampsia with severe features was defined in the American Congress of Obstetricians and Gynecologists executive summary on hypertension in pregnancy.3 The HELLP syndrome (hemolysis, elevated liver enzymes, low platelets) probably represents a subtype of preeclampsia with severe features and was defined based on either Mississippi or Tennessee criteria.4
In preeclampsia, excessive apoptotic feto-placental debris in the maternal circulation leads to systemic inflammation and angiogenic dysregulation.5–7 The complement system appears to play a pivotal role in this systemic inflammation. Excessive complement activation can result from endothelial damage caused by angiogenic imbalance. Animal and human studies have suggested that inhibiting the complement system may mitigate and reverse renal pathology associated with preeclampsia.8–12
This case report highlights the potential benefit of complement inhibition to treat preeclampsia with severe features that developed in the postpartum setting.
Case presentation
A 29-year-old woman G3P2002 was diagnosed with preeclampsia at 36 weeks gestation based on persistently elevated blood pressure and proteinuria (urine protein to creatinine ratio (PCR) 1005.5 mg/g). Given her mild clinical findings, it was elected to defer delivery. She did not return to medical attention until 40 weeks gestation with ruptured amniotic membranes, meconium staining and fetal bradycardia. Her blood pressure was persistently greater than 170/100 mm Hg.
She received intravenous magnesium. Emergency caesarian section was performed with delivery of a
viable neonate. The delivery was complicated by excessive bleeding, resulting in transient intraoperative hypotension. A massive transfusion protocol was initiated, with stabilisation of blood pressure. Vasopressors were not required.
She was transferred to the ICU. Two days post-delivery, she was noted to have oliguria and AKI. A nephrology consultant noted that she was requiring mechanical ventilation with high oxygen requirements and hypertension was being managed with labetalol. The abdomen was firm, distended and diffusely tender. There was bilateral lower extremity oedema.
Laboratory evaluation indicated deterioration of kidney and liver function, thrombocytopenia and evidence of microangiopathic changes on blood film (tables 1 and 2). Urine microscopy showed microscopic hematuria, pyuria and innumerable muddy brown casts. The urine PCR rose to 10 g/g. Complement levels were reduced (table 1).
Table 1.
Laboratory parameters on admission
| Urinalysis | Cloudy, large blood, protein >1000 mg, leucocyte esterase negative, nitrite negative. |
| Urine microscopy | Leucocyte 20–50/hpf, red blood cell 21–50/hpf, innumerable muddy brown casts. |
| Urine protein to creatinine ratio (≤ 84 mg/g) | 10 068 |
| Leucocyte count (3.9–10.6x109/L) | 14.9 |
| Haemoglobin (125–155 g/L) | 109 |
| Platelets (150–440x109/L) | 198 |
| Reticulocyte (0.5%–1.5%) | 2.2 |
| Haptoglobin (34–200 mg/dL) | <20 |
| Peripheral blood smear | Spheroctytes, 2–3 schistocytes/hpf |
| Prothrombin time (11–14 s) | 13.9 |
| Interntional Normalized Ratio (INR) (0.9–1.1) | 1.2 |
| Partial thromboplastin time (20–40 s) | 36.5 |
| Fibrinogen (150–350 mg/dL) | 147 |
| Blood urea nitrogen (8–21 mg/dL) | 20 |
| Creatinine (0.5–1.2 mg/dL) | 1.1 |
| Albumin (3.5–5.0 g/dL) | 1.8 |
| Aspartate aminotransferase(5–30 U/L) | 2593 |
| Alanine aminotransferase(5–30 U/L) | 2184 |
| Lactate dehydrogenase, blood (50–150 U/L) | 5064 |
| Total bilirubin (0.1–1.2 mg/dL) | 1.4 |
| Complement C3 (90–180 mg/dL) | 43 |
| Complement C4 (10–40 mg/dL) | 6.6 |
| Complement C6 (32–57 mg/dL) | <4 |
| Complement total, CH50 (42–60 U/mL) | 19 |
| Erythorcyte sedimentaion rate (ESR) (10–15 mm/h) | 79 |
| C reactive protein (CRP) (<5 mg/L) | 214 |
| A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS 13 activity) | Normal |
| Antinuclear antibody titer | Homogenous, 1:80 |
| Anti-Sjögren’s-syndrome-related antigen SSA/SSA Indx (≤99) AU/mL) | Negative/13 |
| Anti-Sjögren’s-syndrome type B SSB/SSB Indx (≤99) AU/mL) | Negative/8 |
| Anti-Smith antibody SM/SM Indx (≤99) AU/mL) | Negative/10 |
| anti-U1 ribonucleoprotein RNP/RNP Indx (≤99) AU/mL) | Negative/9 |
| dsDNA/dsDNAIndx (≤99) IU/mL) | Negative/8 |
| Centromere Indx (≤99) AU/mL) | Negative/5 |
| Lupus anticoagulant | Negative |
| AnticardiolIpin IgG (≤19.9) | 3.2 |
| Anticardiolipin IgM (≤19.9) | 6.3 |
Laboratory values in parentheses depict the normal range for that test.
Chest X-ray revealed pulmonary vascular congestion. Ultrasound demonstrated an intra-abdominal fluid collection anterior to the liver. Abdominal CT revealed a subcapsular haematoma in the right hepatic lobe.
Differential diagnosis
Thrombotic thrombocytopenic purpura.
Atypical haemolytic uremic syndrome.
Systemic lupus erythematosus.
Anti-phospholipid syndrome.
Preeclampsia/HELLP.
Treatment
Despite adequate resuscitation and stabilisation of blood pressure, she remained oliguric with progressive worsening of renal function. This led to initiation of continuous venovenous hemodiafiltration and subsequent transitioning to intermittent hemodialysis.
As the differential diagnosis included both preeclampsia with severe features and primary thrombotic microangiopathy (TMA) (ie, TTP), plasma exchange was administered for four consecutive days using fresh frozen plasma as replacement fluid. There was no improvement in haematologic or renal function following plasma exchange.
On hospital day 5, she received a single dose of eculizumab 900 mg.
Outcome and follow-up
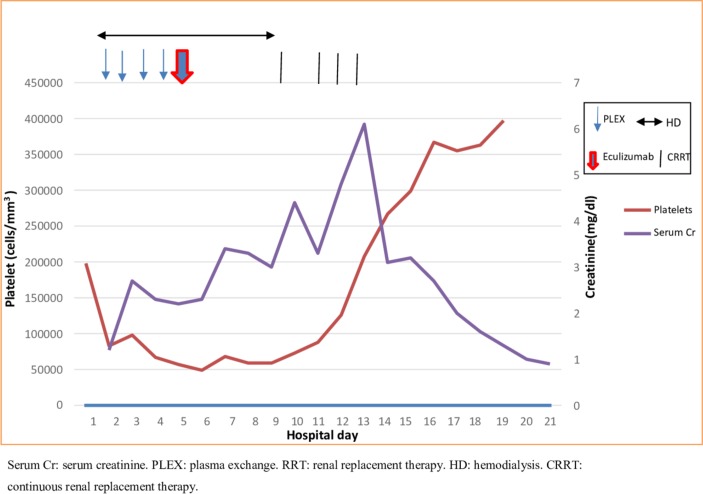
Following treatment with eculizumab, there was a gradual clinical improvement. Within 3 days of administration, she was extubated and labetalol was switched to oral administration. At 7 days post eculizumab, there was improvement in liver function tests and lactate deyhydrogenase (LDH), and normalisation of haematologic parameters and complement levels (table 2). Renal function was improved and hemodialysis discontinued (figure 1). Two weeks post eculizumab, blood pressure was normal, and liver and kidney function had normalised. Urine PCR had improved (now 2.6 g/g).
Table 2.
Trend of hemolytic markers, hepatic and kidney functions, complement levels and proteinuria in relation to plasma exchange and eculizumab therapy during hospitalisation until 1 week post discharge
| Laboratory test | Hospital day 1 | Day 2 (Renal Consult) |
Day 4 (Post-plasma exchange) |
Day 7 (2 days post-eculizumab) |
Day 14 (9 days post-eculizumab) |
Day 22 (Date of discharge) |
Day 29 (A week post- discharge) |
| Haemoglobin (125–155 g/L) | 109 | 82 | 58 | 77 | 82 | 83 | 111 |
| White cell count (3.9–10.6x109 /L) | 14.9 | 10.6 | 8.3 | 11.4 | 12.9 | 6.8 | 6.6 |
| Platelet count (150–440x109/L) | 198 | 83 | 35 | 59 | 260 | 403 | 364 |
| Blood urea nitrogen (8–21 mg/dL) | 20 | 20 | 34 | 67 | 60 | 21 | 15 |
| Creatinine (0.5–1.2 mg/dL) | 1.1 | 1.4 | 3.1 | 3.2 | 3.1 | 1.0 | 0.9 |
| Total bilirubin (0.1–1.2 mg/dL) | 1.4 | 2.1 | 2.6 | 4.6 | 3.1 | 1.5 | 1.5 |
| Alkaline phosphatase(30–115 U/L) | 111 | 94 | 171 | 238 | 308 | 310 | 225 |
| Aspartate aminotransferase (5–30 U/L) | 2593 | 4211 | 8974 | 170 | 64 | 49 | 36 |
| Alanine aminotransferase (5–30 U/L) | 2184 | 2976 | 6572 | 175 | 71 | 39 | 26 |
| Lactate dehydrogenase, blood (50–150 U/L) | 5064 | 5484 | 6572 | 1879 | 1460 | 1135 | |
| Haptoglobin (34–200) mg/dL | <20 | ||||||
| Reticulocyte count (0.5%–2.0%) | 2.2 | 9.3 | |||||
| Complement C3 (90–180 mg/dL) | 43 | 63 | 127 | ||||
| Complement C4 (10–40 mg/dL) | 6.6 | 11.9 | 27.5 | ||||
| CH50 (42–60 U/mL) | 19 | 50 | |||||
| Urine protein to creatinine ratio (≤84 mg/g) | 1005 | 10 068 | 2618 | 2718 |
Figure 1.
Platelet and Cr trends related to PLEX, eculizumab and RRT.
Analysis of common gene mutations associated with complement-mediated TMA revealed no abnormalities (table 3).
Table 3.
Atypical Hemolytic Uremic Syndorme genetic testing panel
| CFH | Negative |
| C3 | Negative |
| CFB | Negative |
| CFI | Negative |
| MCP | Negative |
| CFHR1/CFHR3 | Negative |
| DGKE | Negative |
| THBD | Negative |
CFH, complement factor H; CFB, complement factor B; CFI, complement factor I; CFHR1/CFHR3, complement factor H related protein 1 and 3; MCP, membrane cofactor protein; DGKE, diacylglycerol kinase-ε; THBD, thrombomodulin.
Discussion
Preeclampsia is a multi-system progressive disorder characterised by new onset hypertension and proteinuria (protein excretion of >300 mg/24 hours), or hypertension and end-organ dysfunction with or without proteinuria, after 20 weeks of gestation or postpartum in previously normotensive women.3 Its pathogenesis has not been fully clarified. Delivery of the fetus and placenta is the only curative treatment, which carries risks of prematurity and its associated complications.
Current evidence suggests the role of complement system activation in the pathogenesis of preeclampsia.8–12 In a normal pregnancy, the ongoing complement activation is strictly regulated by membrane regulators decay-accelerating factor, membrane cofactor protein and CD59 located on the trophoblast cells of the placenta. A highly activated complement response that escapes the regulatory mechanism can lead to adverse consequences, such as preeclampsia or recurrent miscarriages.9
Derzsy et al found that healthy pregnant women had significantly higher complement activation proteins level such as C4d, C3a, soluble membrane attack complex (sC5b-9), C3, C9, factor H and C1 inhibitor, as opposed to non-pregnant women.9 The levels of C4d, C3a, C5a and C5b-9 were increased in pre-eclamptic women as compared with normal pregnancies. Additionally, low C3 levels were reported in the pre-eclamptic women.9 10 Recently, increased activity of the alternative complement pathway (ACP) was found in HELLP serum compared with normal pregnancy and non-pregnant controls using modified Ham test.12
The kidney involvement in preeclampsia is attributed to glomerular endothelial damage, which is due to an imbalance between pro-angiogenic and anti-angiogenic factors.13 14 An increase in the anti-angiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1) can prevent the vascular endothelial growth factor from maintaining the renal endothelium, leading to endothelial damage.14 15 Injured fenestrated glomerular endothelium can activate the complement cascade.16 Penning et al demonstrate that the glomeruli of pre-eclamptic women stained predominantly positive for C4d deposits, a marker of complement activation, In contrast, C4d deposits were significantly less present in non- hypertensive pregnant women and non-pregnant women with chronic hypertension groups.17 This supports the role of the complement activation in initiating and/or maintaining renal damage in preeclampsia.
Eculizumab, a monoclonal antibody inhibitor of C5, reduces the generation of complement components C5a and C5b-9 and their downstream effects. Eculizumab appears to be safe in pregnancy as demonstrated by its use in paroxysmal nocturnal hemoglobinuria18 19 and atypical hemolytic uremic syndrome (aHUS).19–21
Burwick et al administered eculizumab to a 35-year-old primigravida at 26 weeks gestation with severe preeclampsia and the HELLP syndrome. The patient experienced clinical improvement with normalisation of liver enzymes and platelet levels.22
In this case report, there was postpartum worsening of preeclamsia/HELLP leading to multi-organ failure, including AKI and requirement for renal replacement therapy. Following failure of plasma exchange, the patient received a single dose of eculizumab (on postpartum day 5) to interrupt complement activation. Within 1 week of administration, there was evidence for improvement in haematologic/hepatic/renal function leading to cessation of renal replacement therapy.
While spontaneous recovery of organ function following delivery cannot be excluded, the case findings suggest that eculizumab administration may have played a role in her recovery. The normalisation of complement levels following treatment with eculizumab is consistent with interruption of complement activation via targeting of C5 by eculizumab.
The differentiation between HELLP and pregnancy-associated atypical hemolytic uremic syndrome (P-aHUS) is often tricky as they share biochemical features.23 Atypical HUS is mainly characterised by microangiopathic haemolytic anaemia (MAHA) and AKI.12 23 Contrarily, this case presented with multi-organ dysfunction including non-cardiogenic pulmonary oedema, subcapsular hepatic haematoma and impaired liver functions in addition to AKI and MAHA, which is more consistent with preeclampsia with severe features/HELLP.
Renal biopsy in our case was not feasible because it would have been hazardous in a thrombocytopenic patient. Histology may show thrombotic microangiopathy and endothelial injury in preeclampsia/HELLP and P-aHUS and not differentiate between the two.
The common genetic mutations associated with the ACP were not detected in our patient, but genetic testing is negative in 40%–50% of patients with aHUS and 20%–36% of HELLP may have mutations.11 24 HELLP and P-aHUS may be part of a spectrum.23
Additional studies are needed to validate this small number of case reports, concomitant with animal research to demonstrate the mechanism of injury. At this point, however, we should be rethinking our approach to treat severe preeclampsia/HELLP.
Learning points.
Complement dysregulation may play a central role in the pathogenesis of preeclampsia/hemolysis, elevated liver enzymes, low platelets (HELLP).
Inhibiting the terminal complement cascade is a potential therapeutic target to treat severe preeclampsia/HELLP either intrapartum or postpartum.
The use of eculizumab, despite its cost, may improve kidney function in Preeclampsia/HELLP and be cost effective by preventing chronic kidney disease and the need to renal replacement therapy.
Footnotes
Contributors: HE and ME contributed to the conception and design, interpretation of data and drafting the article. LS and AA contributed to revising it critically for important intellectual content. All authors contributed to the final approval of the version published; agreed to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Denny KJ, Woodruff TM, Taylor SM, et al. . Complement in pregnancy: a delicate balance. Am J Reprod Immunol 2013;69:3–11. 10.1111/aji.12000 [DOI] [PubMed] [Google Scholar]
- 2. Vasquez DN, Estenssoro E, Canales HS, et al. . Clinical characteristics and outcomes of obstetric patients requiring ICU admission. Chest 2007;131:718–24. 10.1378/chest.06-2388 [DOI] [PubMed] [Google Scholar]
- 3. ACOG. Executive Summary: Hypertension in Pregnancy: American Obstetrics and Gynecology, 2013. [Google Scholar]
- 4. Sibai BM, Taslimi MM, El-Nazer A, et al. . Maternal-perinatal outcome associated with the syndrome of hemolysis, elevated liver enzymes, and low platelets in severe preeclampsia-eclampsia. Am J Obstet Gynecol 1986;155:501–7. 10.1016/0002-9378(86)90266-8 [DOI] [PubMed] [Google Scholar]
- 5. Huppertz B, Kingdom J, Caniggia I, et al. . Hypoxia Favours Necrotic Versus Apoptotic Shedding of Placental Syncytiotrophoblast into the Maternal Circulation. Placenta 2003;24:181–90. 10.1053/plac.2002.0903 [DOI] [PubMed] [Google Scholar]
- 6. Redman CWG, Tannetta DS, Dragovic RA, et al. . Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012;33:S48–54. 10.1016/j.placenta.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 7. Levine RJ, Maynard SE, Qian C, et al. . Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–83. 10.1056/NEJMoa031884 [DOI] [PubMed] [Google Scholar]
- 8. Buurma A, Cohen D, Veraar K, et al. . Preeclampsia is characterized by placental complement dysregulation. Hypertension 2012;60:1332–7. 10.1161/HYPERTENSIONAHA.112.194324 [DOI] [PubMed] [Google Scholar]
- 9. Derzsy Z, Prohászka Z, Rigó J, et al. . Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol 2010;47:1500–6. 10.1016/j.molimm.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 10. Boij R, Svensson J, Nilsson-Ekdahl K, et al. . Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol 2012;68:258–70. 10.1111/j.1600-0897.2012.01158.x [DOI] [PubMed] [Google Scholar]
- 11. Salmon JE, Heuser C, Triebwasser M, et al. . Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE Cohort. PLoS Med 2011;8:e1001013 10.1371/journal.pmed.1001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaught AJ, Gavriilaki E, Hueppchen N, et al. . Direct evidence of complement activation in HELLP syndrome: A link to atypical hemolytic uremic syndrome. Exp Hematol 2016;44:390–8. 10.1016/j.exphem.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karumanchi SA, Maynard SE, Stillman IE, et al. . Preeclampsia: a renal perspective. Kidney Int 2005;67:2101–13. 10.1111/j.1523-1755.2005.00316.x [DOI] [PubMed] [Google Scholar]
- 14. Maynard SE, Min JY, Merchan J, et al. . Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–58. 10.1172/JCI17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eremina V, Jefferson JA, Kowalewska J, et al. . VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008;358:1129–36. 10.1056/NEJMoa0707330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SH, Jeong HJ. Glomerular C4d Deposition Indicates in situ Classic Complement Pathway Activation, but is not a Marker for Lupus Nephritis Activity. Yonsei Med J 2003;44:75–80. 10.3349/ymj.2003.44.1.75 [DOI] [PubMed] [Google Scholar]
- 17. Penning M, Chua JS, van Kooten C, et al. . Classical complement pathway activation in the kidneys of women with preeclampsia. Hypertension 2015;66:117–25. 10.1161/HYPERTENSIONAHA.115.05484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly R, Arnold L, Richards S, et al. . The management of pregnancy in paroxysmal nocturnal haemoglobinuria on long term eculizumab. Br J Haematol 2010;149:446–50. 10.1111/j.1365-2141.2010.08099.x [DOI] [PubMed] [Google Scholar]
- 19. Sarno L, Tufano A, Maruotti GM, et al. . Eculizumab in pregnancy: a narrative overview. J Nephrol 2019;32:17–25. 10.1007/s40620-018-0517-z [DOI] [PubMed] [Google Scholar]
- 20. Ardissino G, Wally Ossola M, Baffero GM, et al. . Eculizumab for atypical hemolytic uremic syndrome in pregnancy. Obstet Gynecol 2013;122:487–9. 10.1097/AOG.0b013e31828e2612 [DOI] [PubMed] [Google Scholar]
- 21. Cañigral C, Moscardó F, Castro C, et al. . Eculizumab for the treatment of pregnancy-related atypical hemolytic uremic syndrome. Ann Hematol 2014;93:1421–2. 10.1007/s00277-013-1970-3 [DOI] [PubMed] [Google Scholar]
- 22. Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 2013;34:201–3. 10.1016/j.placenta.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 23. Shanmugalingam R, Hsu D, Makris A. Pregnancy-induced atypical haemolytic uremic syndrome: a new era with eculizumab. Obstetric Medicine 2018;11:28–31. 10.1177/1753495X17704563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fakhouri F, Jablonski M, Lepercq J, et al. . Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood 2008;112:4542–5. 10.1182/blood-2008-03-144691 [DOI] [PubMed] [Google Scholar]