Abstract
Introduction
Children and young people (CYP) in many high-income settings have poor healthcare outcomes, especially those with long-term conditions (LTCs). Emergency and outpatient hospital service use is increasing unsustainably. To address these problems, the Children and Young People’s Health Partnership (CYPHP) has developed and is evaluating an integrated model of care as part of a health systems strengthening programme across two boroughs of London, UK that are characterised by mixed ethnic populations and varying levels of deprivation. The CYPHP Evelina London model of care comprises proactive case-finding and triage, specialist clinics and transformative education and training for professionals working with CYP. Services are delivered by multidisciplinary health teams with an emphasis on increased coordination across primary, community and hospital settings and integration of physical and mental healthcare that accounts for the CYP’s social context.
Methods and analysis
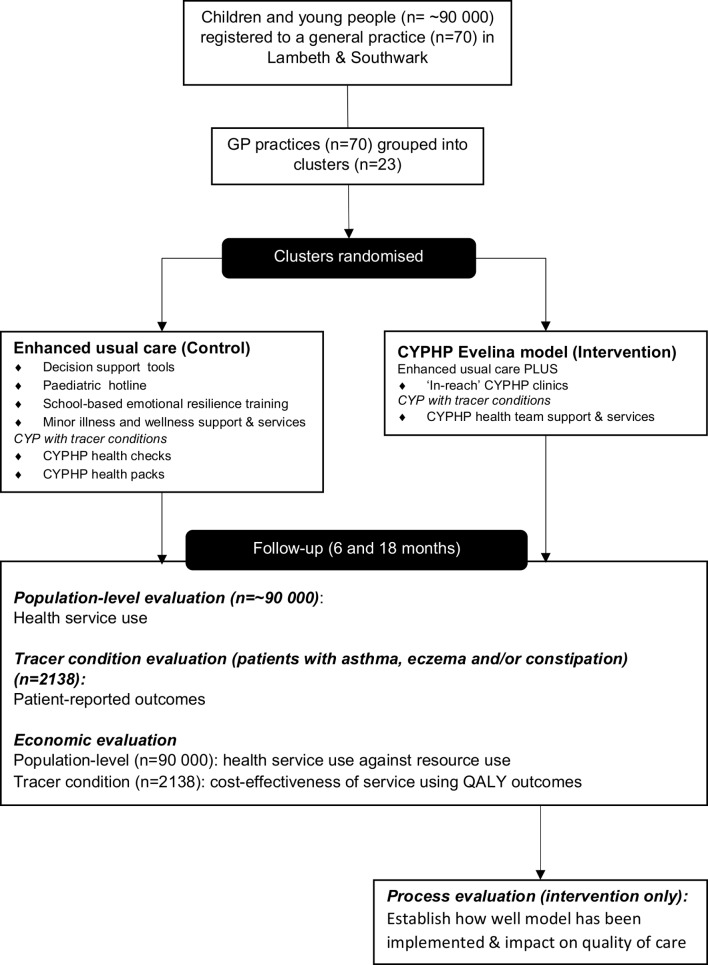
The phased roll out of the CYPHP Evelina London model allows an opportunistic population-based evaluation using a cluster randomised controlled trial design. Seventy general practices across two London boroughs, grouped into 23 clusters, were randomised to provide either the CYPHP model of care (n=11) or enhanced usual care (n=12).
The evaluation will measure the impact of the CYPHP Evelina London model of care on child and parent health and well-being, healthcare quality and health service use up to 2 years postimplementation. A population-level evaluation will use routinely collected pseudonymised healthcare data to conduct a service-use analysis for all CYP registered with a participating general practice (n=~90 000) with the rate of non-elective admissions as the primary outcome. We will seek consent from a subset of this population, with specific conditions (target n=2138) to assess the impact on patient-reported outcomes using the Paediatric Quality of Life Inventory (PedsQL) and Warwick-Edinburgh Mental Well-Being Scale (WEBWMS) as, respectively, the child- and parent-related primary outcomes.
Ethics and dissemination
Ethics approval obtained from South West-Cornwall & Plymouth Research Ethics Committee. Results will be submitted for publication in peer-reviewed journals. Findings will be generalisable to community-based models of care, especially in urban settings. Our process evaluation will identify barriers and enablers of implementation and delivery of care salient to the context and condition.
Trial registration number
NCT03461848; Pre-results.
Keywords: child health, integrated care, cluster randomised controlled trial
Strengths and limitations of this study.
The Children and Young People’s Health Partnership (CYPHP) Evelina London model of care is a new model of integrated, comprehensive, coordinated and tailored care that will be delivered to a population catchment of over 90 000 children and young people across a large and diverse area of South London, UK.
The opportunistic cluster randomised controlled trial design enables unique and rigorous testing of a new model of care, as a population-level health services intervention, for child health in the UK.
Patient-reported and routine service use data will provide information on effectiveness and cost-effectiveness of the CYPHP model of care on outcomes relating to children and young people (CYP) health and well-being, healthcare quality and health services and systems.
Linkage of pseudonymised health service use data will allow population-level impact on patterns of service use to be assessed.
It is anticipated that not all eligible CYP will participate in the intervention or evaluation; our population-based approach to case finding, and recruitment through a patient portal, may present challenges for some patients, for example, with language, literacy or technology barriers. These factors mean that interventions may not reach those most in need. However, we will assess population-level factors, including equity, and through a robust process evaluation that aims to identify barriers and enablers to access the new model of care and gain detailed information about implementation.
Introduction
Approximately 20% of childhood deaths across the USA, England, Australia and New Zealand are thought to be preventable through better clinical care and patient self-management, with higher proportions in specific categories such as children and young people (CYP) with chronic conditions.1 Between 60% and 70% of children who died in the UK between 2001 and 2010 had a chronic condition requiring frequent contact with the health system.2
Chronic, non-communicable disease accounts for 79% of all disability-adjusted life years lost, in young people aged 1–14 years across Europe, with respiratory diseases (mainly asthma), neuropsychiatric disorders, congenital abnormalities and musculoskeletal disorders the predominant causes of morbidity.3 This is mirrored in data from North America and Australia.4 5
The current model of hospital‐centred paediatric care in high-income countries was developed to deliver acute inpatient and high-intensity specialist services, rather than high-quality care for CYP with long-term conditions (LTCs) who need multidisciplinary, coordinated planned care to prevent illness and disease complications, and to maximise well-being and developmental potential.6 The current healthcare model, in the context of the wider health and social care system in the UK, has resulted in suboptimal health outcomes for both acute and chronic illness.7 8 Finally, current services are not as responsive to families’ needs as they should be, and are often inefficient with a reliance on high‐cost emergency department attendance and acute admissions.3 6 9 This is mirrored by inefficiencies seen in other high-income countries.10 There is an urgent need to develop new evidence-based, cost-effective and sustainable healthcare services to meet the increasing demands caused, in part, by the rising prevalence of chronic illness across the life course.3 11–13
The Children and Young People’s Health Partnership (CYPHP) Evelina London model was conceived in response to the evolving healthcare needs of CYP, and the dearth of evidence for health service commissioners and planners on how to address these needs. The CYPHP Evelina London model is an innovative approach to reshaping everyday healthcare services, expanding on the principles of integrated care.14 15 CYPHP Evelina London brings together physical and mental healthcare, and delivers services taking into account the social context of the family. It integrates primary and secondary healthcare, and links healthcare with local government efforts to improve the wider determinants of health. A major focus of the CYPHP Evelina London model is improving frontline care for all CYP. This is vital as primary care and accident and emergency departments are where the majority of healthcare is delivered in the UK context, and act as the gateway to other services. Frontline care can therefore be an enabler or barrier for the rest of the system to function well. In particular, effective and efficient urgent care is important to ensure that sufficient resources are available for the planned, proactive, comprehensive care that CYP with LTC need. This evaluation of the CYPHP Evelina London model of care is designed to generate robust evidence on effectiveness and cost-effectiveness of an integrated model of care for CYP when delivered at scale to inform local, national and international service providers and commissioners.
Evaluation overview
The evaluation, a population-based cluster randomised controlled trial (cRCT) with over 90 000 CYP has four component parts: (1) a pseudonymised population-based evaluation for all CYP in participating general practices, (2) an evaluation of patient-reported outcomes from CYP with one of four specific (or ‘tracer’) conditions, (3) a process evaluation and (4) an economic evaluation. The broad evaluation aims are:
To evaluate the impact of the CYPHP Evelina London model of care on the health, healthcare and health service use of CYP; at the population level and for CYP with tracer conditions.
To understand through the process evaluation how and why the CYPHP Evelina London model is effective or ineffective, and to identify contextually relevant strategies for successful implementation as well as practical difficulties in adoption, delivery and maintenance to inform wider implementation.
To assess the costs of delivery and cost-effectiveness of the CYPHP Evelina model of care compared with enhanced usual care (EUC), through the economic evaluation.
Differences in outcomes will be compared (i) between practices delivering the CYPHP model compared with practices delivering EUC up to 2 years’ postimplementation of the service and (ii) before implementation of the model compared with up to 2 years after.
Methods and analysis
Study design
The implementation of the CYPHP Evelina London model of care across Lambeth and Southwark will occur in stages. This phased roll-out allows the application of an opportunistic cRCT, where for the first stage (lasting for approximately 2 years) general practices are randomised to either the full CYPHP Evelina London model (intervention) or EUC (control). The results of this evaluation will inform local decision-makers about whether and/or how to roll out the CYPHP Evelina London model to the EUC general practices.
Population evaluation
A population-level evaluation will use routinely collected, pseudonymised primary and secondary healthcare data to conduct a service use and economic analysis for all CYP registered with a participating general practice. The model will be evaluated at a population level by comparing health service use (i) between CYP from the CYPHP Evelina London model and EUC clusters, and (ii) to historical data within the CYPHP Evelina London model and EUC arms (ie, before-and-after comparison). This before-and-after analysis will allow us to compare the CYPHP Evelina London model with the healthcare offered before any enhanced care was introduced.
Objectives of the population evaluation are:
To compare health service use (including non-elective admissions, emergency department attendance, outpatient appointments, general practice attendances) over time, before-and-after intervention implementation and between the CYPHP Evelina London model and EUC practices.
To examine the impact of sociodemographic determinants, specifically measures of deprivation, on health service use over time and between the CYPHP Evelina London model and EUC practices.
Tracer condition evaluation
A subset of the population with specific conditions (asthma, epilepsy, constipation, eczema) will be invited to consent for follow-up, as part of our tracer condition evaluation, to assess the impact of the CYPHP Evelina London model on patient-reported outcomes. These tracer conditions were chosen as they are examples of long-term and common conditions, which will provide generalisable lessons about improving outcomes through healthcare for CYP with ongoing conditions.
Objectives of the tracer condition evaluation are:
To assess the impact of the CYPHP Evelina London model on CYP’s health-related quality of life (HRQOL), parent-reported disease severity, prevalence and severity of mental health difficulties, and mental well-being among parents over time, before-and-after intervention implementation and compared with EUC practices.
To assess the equity of service access and delivery (activity, costs, outcomes) across socioeconomic backgrounds.
Process evaluation
A nested process evaluation will explore how well the CYPHP Evelina London model has been implemented and its impact on quality of care (eg, patient/family experience, case notes audits, prescribing rates). Objectives relating specifically to the process evaluation and details of methods are presented in our accompanying process evaluation protocol entitled ‘The Children and Young People’s Health Partnership Evelina London Model of Care: Process Evaluation Protocol’.
Economic evaluation
We will assess the cost to the National Health Service (NHS) of delivering the CYPHP Evelina London model, cost savings in relation to any decrease in health service use and cost-effectiveness of the model in terms of utility in relation to HRQOL of CYP with tracer conditions.
Objectives of the economic evaluation are:
To quantify the differences in resource use and costs linked to professional contacts and services delivered in managing the tracer conditions between the CYPHP Evelina London model and EUC.
To assess secondary healthcare contacts and costs to the NHS.
To evaluate cost-effectiveness by combining evidence on cost impacts and HRQOL outcomes for CYP with tracer conditions.
Hypothesis
We hypothesise that patients from both the CYPHP Evelina London model and EUC practices will show improvement in health outcomes between baseline and follow-up up to 2 years postimplementation. However, we hypothesise that the impact on health outcomes will be significantly greater in patients from CYPHP Evelina London practices compared with patients from EUC practices. In addition, we anticipate that savings attributed to service activity reductions at a population level will outweigh the costs of running the service and that the service will be cost-effective at the tracer condition level.
Study setting
The study is being run in two inner-city boroughs of South London in the UK, Lambeth and Southwark. Child health outcomes for these two inner-city boroughs are worse in many instances than average in England, with high and rising accident and emergency attendance rates for CYP, emergency hospital admissions and hospital outpatient use.16 The CYPHP Evelina model of care components are being rolled out across general practices, schools and hospitals within Lambeth and Southwark.
Interventions
The CYPHP Evelina London model aims to provide comprehensive coordinated care for CYP, and tailored care that is responsive to patients’ needs. In practice, this means integrating primary and secondary healthcare, physical and mental healthcare, healthcare with public health and improving the age appropriateness of care. Providing tailored care that is responsive to patients’ needs will be achieved through roll out of several universal and targeted services, and through health system strengthening initiatives including intrasectoral and intersectoral partnerships, workforce training, technology and analytics. CYPHP intervention functions have been designed to target barriers to effective management of physical, mental and social determinants of health at both a service-provider and patient-level to maximise behaviour change. Further details of the underlying theory and activities involved in the model of care, and the health needs it seeks to address, are described in our model of care paper.17 During phased roll out and the evaluation trial, the CYPHP Evelina London model comprises two groups: (1) interventions that are being implemented across both arms of the trial, called EUC and (2) the full CYPHP Evelina London model, comprising EUC plus additional interventions. Thus, EUC serves as the control arm, and the full CYPHP Evelina London model serves as the intervention arm. Services include care for CYP and support for parents and general practices; described in detail below.
Enhanced usual care (control arm)
All practices within Lambeth and Southwark will receive:
Decision support tools for general practices comprising guidelines (in line with national evidence-based guidelines), algorithms and referral guidance for common conditions such as constipation, eczema, urinary tract infection, enuresis, headache and food allergies. They are in an electronic format, embedded into local general practice data systems so that they can be accessed easily during a consultation.
Paediatric hotline enabling rapid communication between general practices and paediatricians to discuss urgent support, management or referral of an individual child or young person.
School-based emotional resilience building and mental health first aid.
Minor illness and wellness support and services for the most common problems and illnesses, to help parents and professionals to keep CYP well at home.
CYPHP Health Checks for CYP with tracer conditions (asthma, epilepsy, eczema, constipation) and their parents—a biopsychosocial questionnaire which supports tailored care planning.
CYPHP Health Packs for CYP and their parents, comprising self-management support, health promotion and health education material.
Parents of patients with tracer conditions are invited to complete a condition-specific biopsychosocial questionnaire (CYPHP Health Check) about disease or condition status, emotional well-being and social factors. Invitation to complete the Health Check will happen by one of four methods. First, eligible families are identified by their general practice and sent a letter and text messages that invites them to complete an online CYPHP Health Check. Second, general practices and secondary care sites (eg, specialist clinics, outpatient departments) have paper copies of the Health Check available with prepaid envelopes. Third, patients may self-direct to the Health Check web page which is promoted widely, for example, through schools, community events, pharmacists and social media. Finally, healthcare providers may directly refer patients to the service. Information from the CYPHP Health Check will be added to patients’ general practice records, and families will be sent a summary of their scores on the questionnaire and a CYPHP Health Pack.
CYPHP Evelina London model (intervention arm)
In addition to the components of the EUC arm, the CYPHP Evelina London model comprises two types of clinical services: targeted care for CYP with ongoing (tracer) conditions, and universal care available for CYP with any condition.
CYP with tracer conditions are eligible for a tailored clinical service delivered by the multidisciplinary CYPHP Health team in primary and community care settings and in patient’s homes. CYP and families complete a CYPHP Health Check which provides information for triaging and tailoring care. The CYPHP Health Team comprises specialist children’s nurses, a children’s pharmacist, mental health workers, associated school nurses and backed up by consultant paediatrician, child and adolescent psychiatrist and general practice. Care includes health promotion, preventive and reactive care, and integrates services both vertically across primary and secondary care and horizontally between sectors.
CYP with any condition are eligible for ‘in-reach’ CYPHP clinics. These clinics are integrated child health clinics jointly run by general practices and local ‘patch paediatricians’ who are linked to a cluster of general practices. Clinics are held in primary care settings. They offer generalist and specialist advice co-located and coordinated conveniently close to home for patients. In-reach clinics will typically be for CYP who would otherwise have been referred to hospital for an outpatient appointment with a general paediatrician. In-reach clinics also aim to improve clinical decision-making, provide shared learning opportunities and through building trust, cooperation and direct and virtual team-working between general practices and patch paediatricians, integrates services vertically across primary and secondary care.
The hypothesised active components of interventions available in each arm have been mapped against the 12 domains of the Theoretical Domains Framework (TDF) to evidence the proposed mechanism through which the intervention becomes effective (table 1). The TDF is a synthesis (from across existing theories) of the different behavioural domains, which interventions may target to influence behaviour change.18 Thus, the TDF is useful for aiding intervention design and for process evaluations that aim to determine whether mechanisms of actions were as anticipated. While some services are available in both arms and are hypothesised to improve outcomes (eg, education and training), we hypothesise patients receiving the CYPHP Evelina London model will have significantly improved outcomes than patients receiving EUC by the additive behavioural domains targeted and the increased intensity through which domains are targeted due to the mode of administration. For example, Health Packs received in EUC target ‘motivation and goals’ by novel goal setting and action planning exercises. However, while this material is delivered passively through written material in EUC, ‘motivation and goals’ will be targeted in patients receiving CYPHP care through goal-based outcome measures for children and nurses being able to talk through the material face-to-face and provide feedback on meeting those goals.
Table 1.
Mapping CYPHP components to the constructs of the Theoretical Domains Framework
| Domain | CYPHP model of care | Enhanced usual care | |||
| CYPHP care for tracer conditions | CYPHP ‘in-reach’ clinics | CYPHP Health Checks for tracer conditions |
Support tools and services for health professionals | Education and training | |
| Knowledge: an awareness of the existence of something | One-to-one appointments where patients can ask specific questions. | One-to-one learning in joint clinics where there is opportunity to learn knowledge. | Health Packs describe to patients the causes and triggers of their condition. | Evidence-based guidelines, algorithms and referral guidance for common conditions (eg, urinary tract infection, headache, allergies). | Training to improve awareness of difficulties within CYP’s health to:
|
| Skills: ability or proficiency acquired through practice |
|
General practices working with consultant to impart skills in managing certain conditions. | Health Packs designed to provide valuable skills-based techniques in managing condition rather than simply provide information. | Training for:
|
|
| Social or professional role and identity: a coherent set of behaviours and displayed personal qualities of an individual in a social or work setting | Multidisciplinary culture of health staff team places emphasis and responsibility on treating social and mental health concerns in addition to focusing on physical condition. | ||||
| Beliefs about capabilities: self-efficacy or acceptance of the truth, reality or validity about an ability, talent or facility that a person can put to constructive use | Encouraging CYP and families to better self-manage the child’s condition. | Teaching other general practices how they can better manage a child’s presentation of illnesses. | |||
| Beliefs about consequences: acceptance of the truth, reality or validity about outcomes of a behaviour in a given situation | Routine visits help encourage positive patterns of behaviour and deter negative patterns of behaviour by providing feedback by health team. | Information about what will happen if CYP do not better manage their condition. | Training on the lasting impact of not treating CYP mental and physical health early to general practices, teachers and personal advisors. | ||
| Motivation and goals: intention or mental representations of outcomes or end states that an individual wants to achieve | Goal-based outcomes used routinely as part of clinical care to help encourage CYP to manage condition for a reason that is salient to them. | Goal setting exercises help CYP realise why managing their condition is relevant. | |||
| Memory attention and decision processes: the ability to retain information, focus selectively on aspects of the environment and choose between alternatives | Clinical templates to aid nurses to talk through physical, mental and social barriers for CYP not self-managing their condition effectively. | Health Pack material for CYP focuses on self-monitoring techniques (eg, take medication, plan for likely triggers). |
|
||
| Environmental context and resources: any circumstance of a person’s situation or environment that discourages or encourages the development of skills and abilities, independence, social competence and adaptive behaviour | CYPHP nurses are flexible to allow some patients home visits so that they can better understand the triggers for poor health symptoms. Appointments also longer to allow time for CYP to express their concerns. | Patients can receive specialist advice, with their general practice, within practices close to home rather than having to go to secondary or tertiary settings. | Resources embedded into local general practice data systems so that they can be accessed easily during a consultation to help general practices provide evidence-based best practice. | ||
| Social influences: those interpersonal processes that can cause individuals to change their thoughts, feelings or behaviours | CYPHP clinics designed to encourage interaction with health professional peers to gain better understanding of condition. | ||||
| Emotion: a complex reaction pattern, involving experiential, behavioural and physiological elements, by which the individual attempts to deal with a personally significant matter or event | CYPHP health team is trained to focus on the emotional impact of the condition and treat with equal emphasis as the physical condition. | Health Pack material has sections focused on techniques to manage mood and emotional concerns. | Clinical templates to guide care place focus on asking about any emotional concerns the CYP may be experiencing. | All training is focused on the emotional concerns of CYP. | |
| Behavioural regulation: anything aimed at managing or changing objectively observed or measured actions | Clinical templates promote standardised way of documenting care delivered and received. | Clinical templates and guidelines provide framework to guide clinical care. | |||
| Nature of the behaviours: description of how the behaviour is conducted | Documented procedures on how to manage the physical, social and emotional concerns of CYP. | Behaviours taught through collaborative clinics will be taken by general practices to use in regular practice. | Visual information on how to conduct positive self-management behaviours. | Guidance on appropriate behaviours to follow in providing support. | Training to discourage maladaptive behaviours and foster new patterns. |
Green, active delivery (eg, face-to-face, guided demonstration).
Yellow, passive delivery (eg, written text, leaflet).
CYPHP, Children and Young People’s Health Partnership; CYP, children and young people.
Study eligibility criteria
For the population-level evaluation, using pseudonymised data, there are very broad eligibility criteria, as the purpose of the evaluation is to include as many CYP as possible. The only criteria are that the CYP is (i) <16 years of age at the time of service roll out and (ii) registered with a participating practice in Lambeth and Southwark. For the tracer condition evaluation, the same eligibility criteria as the population evaluation apply, and in addition CYP must be diagnosed or identified as having one or more of the four tracer conditions (constipation, eczema, epilepsy, asthma), express interest in the study when completing a CYPHP Health Check (described below) and give informed consent (described below).
Participants will be excluded from the evaluation if any of the following applies:
If during the evaluation period, the patient diagnosis changes and a tracer condition no longer applies.
If the patient is no longer registered with a participating practice (of the total 89 practices in Lambeth and Southwark, all are participating except 19 pilot practices).
If the patient moves their primary residence outside of Lambeth or Southwark.
Randomisation and blinding
As part of the implementation of the CYPHP Evelina London model within Lambeth and Southwark, general practices were grouped into virtual clusters. Where possible, clusters were created aligned to general practice Federation ‘neighbourhoods’ or other existing groupings. These primary care practice clusters consist of two to four general practices grouped together to allow the practices to share resources and hold ‘in-reach’ CYPHP clinics with a local ‘patch paediatrician’.
Of the 89 general practices within Lambeth and Southwark, 19 practices took part in pilot testing of some components of the CYPHP Evelina London model of care. As such, these 19 practices were not randomised.
Randomisation was at the level of primary care practice cluster. Seventy general practices were grouped into 23 clusters and were randomised to receive either the CYPHP Evelina London model of care (n=12) or EUC (n=11). Clusters were initially stratified by borough. A restricted randomisation was then carried out on the 23 clusters. Restriction ensured minimal difference between intervention and control arms with regard to:
Baseline Index of Multiple Deprivation (IMD): difference in mean IMD score <2.5 (mean IMD 30, range of IMD mean score by cluster 20–37).
Income Deprivation Affecting Children Index (IDACI): difference in mean IDACI <2.5 (mean IDACI 29, range 17–37).
CYP population under-16 per general practice cluster: difference in mean population <1000 (mean under-16 population 3914, range 2951–5674).
Outpatient (OP) clinic referrals: difference in mean number of referrals <100 (mean number of OP referral 373, range 256–505).
We generated 56 580 unique randomisations which met the restriction criteria. We checked that cluster pairs were not always grouped together. From these 56 580 randomisations we selected one at random.
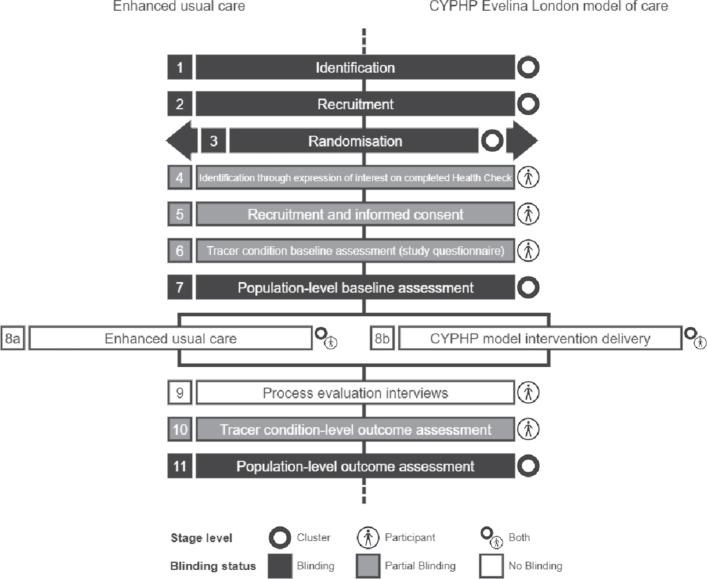
The evaluation will not be blinded at the level of the service delivery or participant. Study personnel are blinded to allocation at the time of recruitment and assessment. Stages of identification, recruitment, randomisation and assessment are highlighted in figure 1 using a cluster trial timeline diagram.19
Figure 1.
Timeline of cluster randomised controlled trial process. CYPHP, Children and Young People’s Health Partnership.
Recruitment and consent
For the population evaluation, data sharing agreements have been established for access to pseudonymised data for all CYP across the two boroughs. Individual-level recruitment and consent is not required for the population evaluation since administrative data are provided to the research team in pseudonymised form. The data are termed pseudonymised as it is only identifiable by a third party (data custodian) who has access to the ‘pseudonymisation key’ which allows record linkage.
For the tracer condition evaluation, at completion of the Health Check, parents of CYP with a tracer condition will be provided with written information for both the parent and CYP about the evaluation and invited to participate in the evaluation. The informed consent process to participate in the evaluation and follow-up can take place through the web-based portal, in person, or by post. Parents will be asked to: (i) provide informed consent for the evaluation team to access their child’s clinical details including Health Check information, and have access to, and link, the child’s general practice and hospital data to assess the impact of CYPHP on both primary and secondary health service use, (ii) complete an evaluation questionnaire at baseline (including health-related quality of life measured by Paediatric Quality of Life Inventory (PedsQL) and Child Health Utility 9D (CHU-9D), and parental well-being measured by the Warwick-Edinburgh Mental Well-Being Scale (WEMWBS)) and (iii) give informed consent to be contacted to participate in qualitative studies evaluating the service. Information sheets will make it clear that parents can consent or refuse consent to any of these components. Participants will be free to withdraw consent without prejudice at any time. A parent/carer alone or with their child may be involved in the recruitment process. If the CYP is under 12 years of age the parent/carer will be asked to provide, on behalf of the child, informed consent, if they are happy to take part in the evaluation. If the CYP is between 12 and 16 years of age, the parent will be asked to provide informed consent and if the CYP is available at the time when parental consent is requested, the CYP will be asked to provide assent if they wish to participate. Questionnaire data for patients with epilepsy is not eligible for the primary comparison between intervention and EUC practices because these patients are primarily managed under secondary care and are found through a different case finding procedure. As such, their experiences of the CYPHP Evelina model of care may be different than the other three conditions. However, their questionnaire data will be used for a before-and-after, epilepsy-specific comparison and they are still included in the process evaluation so that we can understand their experience of care (figure 2). To compensate parents/carers for their time in completing the questionnaires, they will be provided with a £5 gift voucher on completion of the baseline and final follow-up assessments. In addition, following completion of the second assessment, participants will be enrolled into a draw for a tablet computer. Details of recruitment of participants for the process evaluation are outlined in the accompanying paper entitled ‘The Children and Young People’s Health Partnership (CYPHP) Evelina London Model of Care: Process Evaluation Protocol’.
Figure 2.
Diagram of patients, services and levels of the evaluation. CYPHP, Children and Young People’s Health Partnership; CYP, children and young people; QALY, quality-adjusted life year.
Follow-up
Participants who consent to take part in the tracer condition evaluation will be followed up for 2 years and will be asked to complete two questionnaires about the health of their child during the follow-up period. Questionnaire completion may occur up to 4 months after the end of the follow-up period.
Outcomes
Outcome measures include parent-reported child health, health service use and economic impact. Process outcomes, including quality of care, are described in the accompanying process evaluation protocol entitled ‘The Children and Young People’s Health Partnership Evelina London Model of Care: Process Evaluation Protocol’. The methods to assess outcomes are both quantitative and qualitative. ‘Self’-reported outcomes collected include parent-reported and child-related, and parent-related and child self-reported outcomes, where appropriate. Self-reported outcomes will be completed at baseline and up to 2 years’ postimplementation of the service. For all outcomes, differences in outcomes will be compared between practices delivering the CYPHP Evelina London model compared with practices delivering EUC, up to 2 years’ postimplementation of the service. In addition, the impact of the CYPHP Evelina London model will be assessed by comparing outcomes before implementation of the model compared with up to 2 years after.
Population evaluation outcomes
The primary outcome of the population evaluation is the difference in the rate of non-elective hospital admissions (count per patient-year) among CYP from practices delivering the CYPHP Evelina London model compared with practices delivering EUC. Secondary outcomes of the population evaluation will be rates of primary and secondary health service use, including general practice attendances, emergency department attendance, outpatient appointment referrals, outpatient appointment attendances, ambulatory care sensitive admissions, proportion of non-elective admissions that are ambulatory care sensitive and rate (sum per patient-year) of non-elective admissions and outpatient appointment referrals, combined. Box 1 lists the indicators of healthcare use that will be measured using routinely collected health services data, in pseudonymised format.
Box 1. Population evaluation outcome measures.
Primary outcome:
Rate of non-elective admissions.
Secondary outcomes:
General practice attendances.
Emergency department attendances.
Outpatient appointment referrals.
Outpatient appointment attendances.
Ambulatory care sensitive admissions.
Proportion of non-elective admissions that are ambulatory care sensitive.
Rate (sum per patient-year) of non-elective admissions and outpatient appointment referrals.
Tracer condition evaluation outcomes
The primary outcome measure of the tracer condition evaluation is HRQOL, as measured by PedsQL.20 The PedsQL is a brief, standardised, generic assessment instrument that systematically assesses patients’ and parents’ perceptions of HRQOL in paediatric patients. The PedsQL is based on a modular approach to measure HRQOL and consists of a 15-item core measure of global HRQOL and 8 supplemental modules assessing specific symptom or treatment domains. The survey integrates generic core scales and disease-specific modules.
Secondary outcomes of the tracer condition evaluation include health service use, physical condition symptom severity, mental health and parental well-being. Health service use will be analysed using individual data with consent, and aggregate pseudonymised data. Consent will be requested to link patient-level primary and secondary healthcare use data to analyse the impact of the CYPHP Evelina London model on both primary and secondary health service use. In addition, pseudonymised data on healthcare use will be aggregated for all CYP with tracer conditions allowing analysis of the impact of the model on all patients in this population. A further benefit in using pseudonymised data is that it will help to characterise (but not identify) patients who declined to participate, or did not engage. This will identify distributional equity issues by examining the differential impact on costs and outcomes for different patient and social groupings.
Physical condition symptom severity, mental health and parental well-being will be analysed using data derived from the CYPHP Health Check questionnaires which are used by clinicians for biopsychosocial assessment and tailoring care, and if consent is given data will also be used for evaluation (table 2). The CYPHP Health Check includes a condition-specific disease severity questionnaire for each of the four tracer conditions, the Strengths and Difficulties Questionnaire (SDQ) to measure mental health, and a bespoke measure of social conditions (eg, parental mental health, social deprivation). The SDQ is completed as part of the Health Check to provide an estimate of the prevalence and severity of mental health difficulties of CYP with tracer conditions, as measurement of mental health is not routinely collected by (physical) health services within the UK. Scores on the SDQ is being used as part of clinical practice to assess child mental health symptoms and help tailor care specific to need.21 The SDQ is a standardised screening questionnaire used extensively in mental health research with young people.22 The SDQ consists of 25 questions arranged to create four subscales (measuring emotional symptoms, conduct, hyperactivity and inattention and peer relationship difficulties). The impact supplement will also be completed. A version can be completed by the parent/carer for CYP aged 2–17 years. The Asthma Control Test (ACT) is being used to assess severity of physical symptoms in patients with asthma. The ACT is a self-report measure designed for adults and adolescents 12 years or older.23 The Childhood ACT is used for CYP aged 4–11 years. The ACT has five items asking about patients’ symptoms over the past 4 weeks, which are each scored on a 5-point scale. The Childhood ACT has seven items which use a 5-point scale but where four questions are answered by the child and three questions are answered by the parent/carer using the same 4 weeks’ reference frame. Patients with eczema (or their parents/carers) complete the Patient-Oriented Eczema Measure (POEM).24 The POEM is a tool used for monitoring atopic eczema severity. It focuses on the illness as experienced by the patient. The scale includes seven items with a 1-week reference frame, and produces a score (0–28) and severity level (‘clear or almost clear’ to ‘very severe eczema’). Patients with constipation and/or epilepsy (or their parents/carers) will be asked to complete bespoke condition-specific measures created for the purposes of the clinical service. Measures were created by CYPHP clinicians and researchers based on National Institute for Health and Care Excellence guidelines and clinical utility.
Table 2.
Tracer condition outcome measures used as part of clinical service and study evaluation
| Domain measured | Outcome measure |
| Self-report measures used for clinical service and evaluation (CYPHP Health Check) | |
| Asthma severity | Asthma Control Test |
| Eczema severity | Patient-Oriented Eczema Measure |
| Constipation severity | Bristol Stool Chart |
| Bespoke constipation questionnaire | |
| Epilepsy severity | Bespoke epilepsy questionnaire |
| Mental health concerns | Strengths and Difficulties Questionnaire |
| Social context | Bespoke social screen questionnaire
|
| Self-report measures used for evaluation | |
| Primary outcome: health-related quality of life | Paediatric Quality of Life Inventory |
| Economic data on child quality of life | Child Health Utility 9D |
| Parental well-being | Warwick-Edinburgh Mental Well-Being Scale |
| Health service use (individual-level data linked with consent) | |
| Rate of non-elective admissions | |
| General practice attendances | |
| Emergency department attendances | |
| Outpatient appointment referrals | |
| Outpatient appointment attendances | |
| Ambulatory care sensitive admissions | |
| Proportion of non-elective admissions that are ambulatory care sensitive | |
| Rate (sum per patient-year) of non-elective admissions and outpatient appointment referrals | |
CYPHP, Children and Young People’s Health Partnership.
Additional measures, for evaluation only, are asked of parents/carers who have given their consent. The CHU-9D is a generic measure of quality of life that can be applied to paediatric populations.25 The measure consists of items with preference weights that give utility values for each health state described, allowing the calculation of QALYs for use in cost utility analysis. The scale has nine dimensions and each item is scored on a 5-point scale. The WEMWBS is a 14-item scale of mental well-being, validated for adults. WEMWBS covers subjective well-being and psychological functioning, in which all items are worded positively and address aspects of positive mental health.26
The economic evaluation includes assessment of implementation, and primary care and hospital services, primarily from the NHS perspective. Implementation inputs will be measured through activity logs used to record time, equipment and building space and costed using national and locally relevant unit costs. Resource use and costs of services delivered in primary care will be evaluated through use of CYP contact data with specific professionals and services delivered within primary care settings, combined with national and locally relevant unit costs. Hospital-based service contacts will be identified through linkage between primary care and HES data systems. Appropriate national and local unit costs estimates will be applied to cost hospital service contacts. Quality-adjusted life year (QALY) outcomes relating to acute and non-acute impacts on CYP health and quality of life will be estimated from the CHU-9D measure.
Patient and public involvement
The CYPHP Evelina London model was developed with key stakeholders including CYP, carers, frontline practitioners and providers and health service commissioners. Stakeholders were involved in the development of the theoretical framework for CYPHP, identification of research questions and refining the research methodology. A specific CYPHP patient and public involvement group was developed with CYP and their families and allowed us to consult with regard to all aspects of the evaluation design; including appropriateness of outcome measures, consent procedures and self-management material that was developed as part of EUC.
Sample size calculation
For the population evaluation, pseudonymised data from all CYP (<16 years) within participating practices will be used to analyse the impact of the CYPHP Evelina London model on health service use. Eleven clusters in each arm, and an average of 3800 CYP per cluster, provides over 87% power to detect a reduction of 20% in the rate of non-elective admissions, assuming a coefficient of variation of 0.142, and baseline rate of 56 admissions per 1000 person-years. The number of CYP per cluster is estimated conservatively based on the 89 382 CYP (age 0–15 years) registered in 2015 in the general practices in the 23 randomised clusters. The baseline rate of non-elective admissions and the coefficient of variation were estimated using counts of non-elective admissions per cluster from financial years 2013–14 to 2015–16, and counts of CYP enrolled per cluster during 2013–15. The coefficient of variation used in the sample size calculation was the mean of these three estimates. The rate of non-elective admissions was the total rate estimated by combining data from the three financial years 2013–16.
For the tracer condition evaluation, we hypothesise that the intervention will have an effect on both infant health and parent health but we believe that the mechanisms may be theoretically different and we believe that parental well-being may be a potential mediator. Therefore, we have included both a child-based and parent-based health outcome in our sample size calculations. With 11 clusters in each study arm, the study team will need to recruit a minimum of 1068 CYP with a tracer condition (asthma, constipation or eczema) per arm (total 2138) (see ‘Recruitment and consent’ section for rationale why epilepsy not included in sample size calculation). This number of participants will give the study 90% power to detect a mean minimum clinically important difference (MCID) of 4.5 points (SD 16.5) in the primary outcome tool for child HRQOL (parental-reported PedsQL),20 as used previously with CYP with chronic health conditions such as asthma.27 The intraclass correlation coefficient (ICC) is assumed to be 0.02 based on a study of quality of life in CYP with a related condition, hay fever.28 The between-cluster coefficient of variation in cluster size is assumed to be 0.03 based on the harmonic mean and variance of cluster size derived from general practice registrations. The recruitment target also accounts for a 30% loss to follow-up. In total, there are 23 clusters, 12 in one arm and 11 in the other; as such the outlined sample size underestimates the total power as we have assumed 11 clusters in each arm. This same sample size provides over 90% power to detect a mean MCID of 3 points (SD 8.4) in the parental primary outcome tool, WEMWBS.26 Here, the ICC is assumed to be 0.03, based on pilot data from the Wellbeing in Secondary Education (WISE) trial.29 Again, this allows for 30% loss to follow-up.
Data analysis and reporting
A detailed analysis plan will be finalised before receipt of study data. Findings will be reported according to the Consolidated Standards of Reporting Trials guidelines for cRCTs. Flow charts will show the numbers of clusters, the numbers of CYP recruited and followed up to each time point post recruitment. Balance between CYPHP Evelina London and EUC clusters will be presented for a predefined set of potential confounding factors, and analyses adjusted for any major imbalances. All analyses will take into account the cluster design.30 Summary values (of each outcome) will be presented for each cluster, and for CYPHP Evelina London and EUC groups compared using t-test and Χ2 test for continuous outcomes and binary outcomes, respectively.
Random-effects regression analyses using individual-level data will be used to simultaneously adjust for the clustered design and any imbalances between CYPHP Evelina London and EUC arms; logistic regression models will be used for binary outcomes, Poisson regression for rates (eg, admission rates) and linear regression for continuous outcomes (eg, PedsQL scores). Effect sizes will be presented as ORs for binary outcomes, rate ratios for rates and as mean differences for continuous outcomes; 95% CIs will also be given. Regression analyses will also be used to assess whether the impact of the intervention differs by wealth quintile.
Primary analyses will be intention-to-treat and include all data from participants regardless of their exposure to intervention activities. Per-protocol analyses will also be carried out to examine the impact of the intervention taking into account engagement with the respective clinical services of the universal EUC services and services specific to patients with tracer condition.
Ethics and dissemination
We plan to use the Method for Aggregating the Reporting of Interventions in Complex Studies31 approach to bring together complex data from multiple sources to evaluate this complex intervention. Results will be disseminated through publication in peer-reviewed articles, through presentation at national and international meetings, and via websites including CYPHP programme, partners, funder and sponsor. Results, including a lay summary, will be shared with participants through publicly accessible websites, and participants who gave consent will receive information about their contribution to the evaluation. Participant identifiable data will be removed from all publications.
Supplementary Material
Footnotes
Contributors: JJN was responsible for writing the first draft of the protocol and JF was responsible for drafting the second version (joint first authorship). JJN, JF, MH, SC, CL, ME, R-MS, RL, IW were involved in the study design and in obtaining ethical approvals. RL and IW were responsible for study conception (joint last authorship). All authors commented on the manuscript and agreed with the final version.
Funding: This work was supported by Guy’s and St Thomas’ Charity.
Competing interests: None declared.
Ethics approval: This study was approved by South West-Cornwall & Plymouth Research Ethics Committee and the NHS Health Research Authority on 14 December 2017.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Fraser J, Sidebotham P, Frederick J, et al. . Learning from child death review in the USA, England, Australia, and New Zealand. Lancet 2014;384:894–903. 10.1016/S0140-6736(13)61089-2 [DOI] [PubMed] [Google Scholar]
- 2. Hardelid P, Dattani N, Davey J, et al. . Overview of child deaths in the four UK countries. Child Health Reviews–UK. London: Royal College of Paediatrics and Child Health. 2013. https://www.hqip.org.uk/resource/overview-of-child-deaths-in-the-four-uk-countries/#.W8QuzfZFwaE (accessed 15 Oct 2018).
- 3. Wolfe I, Thompson M, Gill P, et al. . Health services for children in western Europe. Lancet 2013;381:1224–34. 10.1016/S0140-6736(12)62085-6 [DOI] [PubMed] [Google Scholar]
- 4. Liu T, Lingam R, Lycett K, et al. . Parent-reported prevalence and persistence of 19 common child health conditions. Arch Dis Child 2018;103:548–56. 10.1136/archdischild-2017-313191 [DOI] [PubMed] [Google Scholar]
- 5. Miller GF, Coffield E, Leroy Z, et al. . Prevalence and costs of five chronic conditions in children. J Sch Nurs 2016;32:357–64. 10.1177/1059840516641190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mansfield A. BMA board of Science. Growing up in the UK: ensuring a healthy future for our children BMA. 2013. https://www.bma.org.uk/collective-voice/policy-and-research/public-and-population-health/child-health/growing-up-in-the-uk (accessed 15 Oct 2018).
- 7. Wolfe I, Cass H, Thompson MJ, et al. . Improving child health services in the UK: insights from Europe and their implications for the NHS reforms. BMJ 2011;342:d1277 10.1136/bmj.d1277 [DOI] [PubMed] [Google Scholar]
- 8. Cheung R. International comparisons of health and wellbeing in early childhood. 2018. https://www.nuffieldtrust.org.uk/files/2018/1521029143_annex-child-health-international-comparisons.pdf (accessed 15 Oct 2018).
- 9. Viner RM, Blackburn F, White F, et al. . The impact of out-of-hospital models of care on paediatric emergency department presentations. Arch Dis Child 2018;103:128–36. 10.1136/archdischild-2017-313307 [DOI] [PubMed] [Google Scholar]
- 10. Braithwaite J. Changing how we think about healthcare improvement. BMJ 2018;361:k2014 10.1136/bmj.k2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davies SC, Lemer C, Strelitz J, et al. . Our children deserve better: prevention pays. Lancet 2013;382:1383–4. 10.1016/S0140-6736(13)62004-8 [DOI] [PubMed] [Google Scholar]
- 12. Brussels European Union. European commission health and consumers directorate‐general. The 2014 EU summit on chronic diseases: conference conclusions. 2014. https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/ev_20140403_mi_en.pdf (accessed 15 Oct 2018).
- 13. NHS England. Five Year Forward View. 2014. https://www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 15 Oct 2018).
- 14. World Health Organization. Integrated care models: An overview. WHO Regional Office for Europe. 2016. http://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf (accessed 15 Oct 2018).
- 15. Wolfe I, Satherley R, Scotney E, et al. . A systematic review and meta-analysis of integrated and chronic care models to improve child health. Submitted to JAMA Pediatr 2018. [Google Scholar]
- 16. Das R, Forman J, Elsherbiny M, et al. . The relationships between deprivation and hospital service use for children and young people locally and nationally: Learning from research to inform innovative service delivery and tackle inequalities Presented at HERON Conference: London, England, 2018. [Google Scholar]
- 17. Wolfe I, Lemer C, Heys M, et al. . New integrated care models to improve health, healthcare quality, and patterns of service use among children and young people Presented at Public Health England Conference. Warwick, England, 2018. [Google Scholar]
- 18. Michie S, Johnston M, Abraham C, et al. . Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26–33. 10.1136/qshc.2004.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caille A, Kerry S, Tavernier E, et al. . Timeline cluster: a graphical tool to identify risk of bias in cluster randomised trials. BMJ 2016;354:i4291 10.1136/bmj.i4291 [DOI] [PubMed] [Google Scholar]
- 20. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126–39. 10.1097/00005650-199902000-00003 [DOI] [PubMed] [Google Scholar]
- 21. Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry 1997;38:581–6. 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- 22. Meltzer H, Gatward R, Corbin T, et al. . The mental health of young people looked after by local authorities in England. London: The Stationery Office, 2003. [Google Scholar]
- 23. Nathan RA, Sorkness CA, Kosinski M, et al. . Development of the asthma control test. J Allergy Clin Immunol 2004;113:59–65. [DOI] [PubMed] [Google Scholar]
- 24. Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients' perspective. Arch Dermatol 2004;140:1513–9. 10.1001/archderm.140.12.1513 [DOI] [PubMed] [Google Scholar]
- 25. Stevens K. The development of a preference based paediatric health related quality of life measure for use in economic evaluation: The University of Sheffield, 2008. [Google Scholar]
- 26. Tennant R, Hiller L, Fishwick R, et al. . The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5:63 10.1186/1477-7525-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varni JW, Burwinkle TM, Seid M, et al. . The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329–41. [DOI] [PubMed] [Google Scholar]
- 28. Hammersley VS, Walker S, Elton R, et al. . Protocol for the adolescent hayfever trial: cluster randomised controlled trial of an educational intervention for healthcare professionals for the management of school-age children with hayfever. Trials 2010;11:84 10.1186/1745-6215-11-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kidger J, Stone T, Tilling K, et al. . A pilot cluster randomised controlled trial of a support and training intervention to improve the mental health of secondary school teachers and students - the WISE (Wellbeing in Secondary Education) study. BMC Public Health 2016;16:1060 10.1186/s12889-016-3737-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayes RJ, Moulton LH. Cluster Randomised Trials. Boca Raton, FL: CRC Press, 2009. [Google Scholar]
- 31. Thorne K, Jerzembek GS, Cheung W-Y, et al. . MATRICS: a method for aggregating the reporting of interventions in complex studies. Trials 2011;12:A147 10.1186/1745-6215-12-S1-A147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.