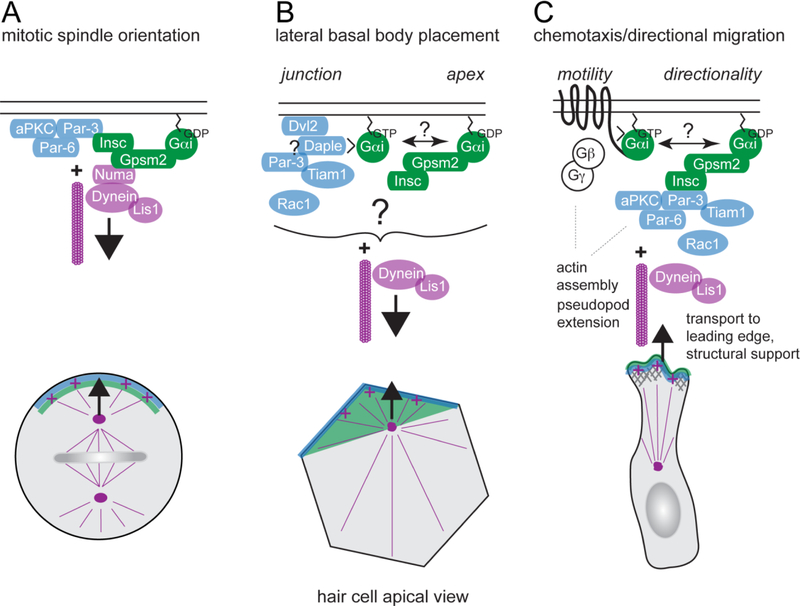
Figure 4. Shared protein modules regulate HC planar polarity and other polarized processes.
A) During oriented cell division, the Gpsm2-Gαi complex, and in some cases the Par3-Par6-aPKC complex, are polarized at the cell cortex and recruit effectors to pull on astral microtubules and position the mitotic spindle. Gαi is myristoylated and anchored at the membrane. Gpsm2-Gαi can interact with the Par3 complex through binding to the Insc adapter, or recruit the minus-end microtubule motor dynein through the Numa adapter. Lis1 is an activator for dynein required for spindle orientation. This evolutionary conserved process controls daughter cell positioning and tissue architecture across multiple tissues. B) During HC planar polarization, dynein-mediated pulling forces on apical microtubules may play a role in the lateral placement of the basal body. Junctional proteins including Dvl2, Daple and Par3, may serve as cortical dynein tethers to anchor pulling forces, and/or stabilize microtubule cortical attachment by activating Rac-Pak signaling. The Insc-Gpsm2-Gαi complex provides a blueprint on the HC apical membrane, and may also plays a role in basal body placement. C) During chemotaxis, for example in neutrophils, motility requires G protein coupled receptors (GPCRs) to sense extracellular cues in the environment and activate heterotrimeric G proteins. Gβγ dissociated from activated, GTP-bound Gαi in turn activates downstream effectors to regulate actin assembly and pseudopod extension at the leading edge. In addition to canonical GPCR signaling, it has been proposed that Gαi-GDP bound to Gpsm2 might more specifically regulate sustained directionality by signaling through Insc and Par3. In other cell types, sustained directionality was shown to require microtubules and Lis1/dynein to regulate transport and polarized activation of signaling molecules such as Rac1 GTPase. It is interesting to speculate that control over Gαi guanine nucleotide exchange involving GEF proteins like GPCRs or Daple might ultimately interconnect different polarity modules that act in concert in HCs.