Abstract
Objective:
Outcome prediction in comatose patients with acute brain injury remains challenging. Regional cerebral oxygenation (rSO2) derived from NIRS (near infrared spectroscopy) is a surrogate for cerebral blood flow and can be used to calculate cerebral autoregulation (CA) continuously at the bedside from the derived cerebral oximetry index (COx). We hypothesized that COx derived thresholds for CA are associated with outcomes in patients with acute coma from neurological injury.
Methods:
A prospective cohort study was conducted in 88 acutely comatose adults with heterogenous brain injury diagnoses who were continuously monitored with COx for up to three consecutive days. Multivariable logistic regression was performed to investigate association between averaged COx and short (in-hospital and 3 month) and long-term (6 month) outcomes.
Results:
Six month mortality rate was 62%. Median COx in non-survivors at hospital discharge was 0.082 [IQR 0.045-0.160] compared to 0.042 [IQR −0.005 to 0.110] in survivors (P=0.012). At 6 months, median COx was 0.075 [IQR 0.27-0.158] in non-survivors compared to 0.029 [IQR −0.015 to 0.077] in survivors (P=0.02). In the multivariable logistic regression model adjusted for confounders, average COx ≥0.05 was associated with both in-hospital mortality (adjusted OR=2.9, 95% CI=1.15-7.33, P=0.02), mortality at 6 months (adjusted OR=4.4, 95%CI=1.41-13.7, P=0.01), and severe disability (modified Rankin Score ≥4) at 6 months (adjusted OR=4.4, 95%CI=1.07-17.8, P=0.04). Area under the ROC curve for predicting mortality and severe disability at 6 months were 0.783 and 0.825, respectively.
Conclusions:
Averaged COx ≥0.05 is independently associated with short and long-term mortality and long-term severe disability in acutely comatose adults with neurological injury. We propose that COx ≥0.05 represents an accurate threshold to predict long-term functional outcome in acutely comatose adults.
Keywords: cerebral oximetry, cerebral perfusion, near-infrared spectroscopy, cerebral autoregulation
INTRODUCTION
Advancements in neurocritical care monitoring have led to improved outcomes for patients with acute neurological injury.1 Cerebral autoregulation (CA) monitoring is one such development that may not only provide prognostic information but also allow for individualization of cerebral perfusion pressure (CPP) targets, which have been associated with improved functional and mortality outcomes.2–6
For the most part, CA monitoring tools such as intracranial pressure (ICP) and brain tissue oxygen partial pressure (PbtO2) monitors are invasive. Over the last decade non-invasive near-infrared spectroscopy (NIRS) measured regional cerebral oxygenation (rSO2) has been evaluated as a surrogate for cerebral blood flow (CBF) for CA monitoring.7, 8 rScO2 represents an indicator of cerebral O2 supply versus demand. Over short periods of time many determinants of cerebral O2 balance, such as cerebral metabolic rate, O2 saturation, and hemoglobin concentration, are stable in the low frequencies associated with CA (i.e. 0.003 to 0.04 Hz).9 The cerebral oximetry index (COx) is calculated as the moving correlation between slow waves of rScO2 and mean arterial pressure (MAP).10 The many advantages of this technology include feasibility for continuous monitoring, no requirement for special expertise, and negligible risk for infectious or hemorrhagic complications.11 In laboratory and clinical investigations, COx has been found to have good correlation with conventional invasive CA measurements derived from PbtO2 (brain tissue oxygen pressure reactivity index),12 and ICP (pressure reactivity index [PRx]) measurements. 13, 14 We have previously reported good correlation and agreement between TCD derived autoregulation measurements – i.e. the mean velocity index (Mx) calculated as the coefficient between slow waves of TCD-measured CBF velocity and MAP - and COx in a similar, but smaller, cohort of adult comatose patients with acute neurologic injury,15 and in patients undergoing cardiac bypass.16 We now hypothesize that COx derived thresholds can be determined, and are significantly associated with neurologic outcomes in patients with acute coma from neurological injury.
Several groups have investigated the relationship between neurologic outcomes from severe neurologic disease and various autoregulation-related indices.15, 17 Both PRx and Mx were found to be significantly associated with 3-month clinical outcomes, even in mixed cohorts of neurocritical care patients with both traumatic and non-traumatic causes of brain injury.17 At this time there is limited evidence to support the ability of NIRS to predict outcome after brain injury,18 although several cohort studies suggest that NIRS-based monitoring has predictive accuracy for outcomes in patients with traumatic brain injury and subarachnoid hemorrhage.19, 20 The primary aim of the present study was to investigate the association of NIRS-based measurements at the bedside with short and long-term outcomes in comatose patients with different types of brain injury, and identify their most accurate thresholds.
METHODOLOGY
Study design
A prospective observational cohort study was conducted in our neuroscience intensive care unit from 2013 to 2017. Acutely comatose patients were monitored continuously for up to 3 days to calculate CA indices at the bedside starting within the first 12 to 48 hours from coma onset. Patients who underwent withdrawal of life support within 48 hours from admission were excluded. Other exclusion criteria included unresponsiveness not primarily due to brain injury (i.e., aphasia, sedation or residual anesthesia, metabolic causes). The protocol was approved by the Johns Hopkins Medical Institutions Review Board; written informed consent was not required due to low risk and standard usage of this type of non-invasive monitoring.
Patients
Coma status was defined as Glasgow Coma Scale (GCS) score ≤ 8. Only patients who had an indwelling arterial catheter placed for clinical indications for direct arterial pressure monitoring were enrolled in the study. Arterial blood pressure (ABP) was monitored continuously with pressure transducers leveled at the heart. Additional variables included demographic characteristics, partial pressure of carbon dioxide (PaCO2), drugs used for sedation, hemoglobin concentration, modified Rankin Scale (mRS) score on admission, and clinical outcomes including mortality and mRS during the index hospitalization, and at 3 and 6 months after hospital discharge. The primary outcome was blinded assessment of mortality at hospital discharge, and at 3 and 6 months. The secondary outcome was blinded assessment of severe disability (defined as mRS ≥4) at hospital discharge and at 6 months. MRS at 3 and 6 months were obtained by telephone interview using the validated telephonic mRS questionnaire21 and if not available, by reviewing the medical records for neurology or neurosurgery clinic visits within 30 days of this time-point.
NIRS-based autoregulation monitoring
The patients were connected to an NIRS monitor (INVOS™ 5100, Covidien, Boulder, CO) using self-adhesive sensors placed on each side of the forehead. Analog MAP signals were obtained from the patient clinical hemodynamic monitor (General Electric, Solar 8000i) and then processed with a DT9800 data acquisition module (Data Translation Inc, Marlboro, MA). Digital NIRS signals were directly collected to a laptop computer and then analyzed using the ICM+ software (University of Cambridge, Cambridge, United Kingdom), as described previously.22, 23 The MAP and NIRS signals were filtered to focus on the frequency of slow vasogenic waves, which have been shown to be a valid objective measure of CA.3, 22, 24 Slow waves are produced by changes in CBF and cerebral blood volume, and believed to occur in response to fluctuations in CPP or to local metabolic changes.25 It has been shown that slow fluctuations in cerebral oximetry as detected by NIRS coincide with, and are implicated in the origin of, ICP slow waves; therefore NIRS may be used as a non-invasive marker of increased ICP slow waves.25 The signals were filtered as non-overlapping 10-second mean values that were time-integrated, which is equivalent to having a moving average filter with a 10-second time window and resampling at 0.1 Hz, thereby eliminating high-frequency components resulting from respiration and pulse waveforms. A continuous, moving Pearson’s correlation coefficient between changes in MAP and rScO2 was calculated rendering a right and left-sided COx. Intact autoregulation is indicated by a COx approaching zero or negative values (i.e., no correlation between rScO2 and ABP),whereas COx approaching 1 indicates impaired autoregulation. COx and MAP were averaged for the whole recording period (recordings lasted from 1 hour to 3 days after injury), thus obtaining the overall autoregulatory status of a patient during the entire monitoring period. If one side was not recorded (i.e. the cable got disconnected, the sensor moved and stopped recording), then only the recorded side accounted for the calculated COx. The data were cleaned to remove artifacts from movement, etc. before analysis. Monitored COx data was not available to the bedside clinician, therefore outcomes were not affected by the recorded data.
Statistical Analysis
Descriptive characteristics of the sample were analyzed using the statistical software Stata version 13.0 (Stata Corp, College Station, TX). Data were checked for normality with Shapiro-Wilk test and graphically using histograms. Non-normal distribution of the data was observed; therefore the data were analyzed using non-parametric techniques. We obtained standardized mean differences (SMD) of cerebral autoregulation indices (e.g., COx) to evaluate the prediction of dichotomous outcome variables defined as mortality (mRS=6) and poor outcome (mRS≥4). After determining the level of significance of COx for the prediction of both outcomes, we categorized COx by threshold sequential cut-off values (in 0.02 steps) and calculated adjusted Z-score values for age and GCS for each of these threshold values. For each outcome measure, the respective COx threshold returning the highest Z-score was assumed to have the best discriminative value per the method used by Sorrentino et al.26 Additionally, we calculated negative predictive values (NPV) and positive predictive values (PPV) manually using mortality and poor outcome tables at the defined COx thresholds.
Continuous variables were analyzed using t-tests, and the Mann-Whitney U test for non-normally distributed data. Categorical variables were analyzed using Chi square test (or Fisher exact test when appropriate). Multivariable logistic regression was used to analyze the relationship between the NIRS-based indices and functional outcome (mRS) adjusted for GCS at the start of monitoring and age. Covariates for regression models were chosen based on bivariate logistic regression with a significance of p<0.05. All analyses were two-tailed, and significance level was determined by p<0.05. Additionally, we calculated area under ROC curves for prediction of mortality and severe disability at 6 months.
RESULTS
Patient characteristics
One hundred and five comatose patients were enrolled and monitored in the study. We excluded 17 patients (10 who had corrupted recordings that could not be opened for analysis, 6 who regained consciousness in less than one hour, 1 whose coma was due to septic encephalopathy), leaving 88 patients eligible for analysis. The median recording duration was 38 hours [interquartile range (IQR) 20–50]. At hospital discharge, 79 (89%) subjects had severe disability (mRS ≥4) or had died (45 [51%]). At 6 months, mortality rate was 48/73 (65%). Table 1 shows sociodemographic and clinical characteristics of the study population. In the univariable analysis, non-survivors at hospital discharge had higher odds of having suffered brain herniation compared to survivors (42% vs. 9%, P<0.001) and had a lower median GCS at the start of monitoring compared with survivors (5 [IQR 3–7] vs. 7 [IQR 5 – 8], respectively; P=0.004). Forty-six percent of patients were receiving sedatives, such as dexmedetomidine, fentanyl, pentobarbital and propofol, during the monitoring period (table 1); however, there was no significant difference between survivors and non-survivors (p=0.12). There was also no difference in COx in the group that received sedation versus the group that did not, (0.06 + 0.09 vs. 0.07 + 0.09, respectively; P=0.88).
Table 1.
Acutely comatose patients with neurological injury: demographics and clinical characteristics (n = 88)
| Variable | Overall (n=88) | Survivors at hospital discharge (n=43) | Non-survivors at discharge (n=45) | p value |
|---|---|---|---|---|
| Gender (F / M), n | 43/ 45 | 23 / 20 | 20 / 25 | 0.39 |
| Age (Mean ± SD), years | 57.8 ± 14.4 | 57.6 ± 13.6 | 57.9 ± 15.3 | 0.93 |
| Race, n (%) | 0.26 | |||
| African American | 46 (52%) | 24 (54%) | 22 (50%) | |
| Caucasian | 36 (41%) | 15 (34%) | 21 (47%) | |
| Asian | 4 (4.6%) | 3 (7%) | 1 (2.3%) | |
| Hispanic/Latino | 2 (2.3%) | 2 (5%) | 0 (0%) | |
| Coma Etiology, n (%) | 0.07* | |||
| Ischemic stroke | 10 (11%) | 4 (9%) | 6 (13%) | |
| Intracerebral hemorrhage | 28 (32%) | 9 (21%) | 19 (42%) | |
| Aneurysmal subarachnoid hemorrhage | 24 (27%) | 13 (30%) | 11 (24%) | |
| Traumatic brain injury | 11 (13%) | 6 (14%) | 5 (11%) | |
| Status epilepticus | 7 (8%) | 6 (14%) | 1 (2.2%) | |
| Meningitis | 1 (1.1%) | 1 (2.3%) | 0 (0%) | |
| Hypoxic ischemic encephalopathy | 2 (2.3%) | 0 (0%) | 2 (4.4%) | |
| Ventriculitis | 1 (1.1%) | 0 (0%) | 1 (2.2%) | |
| Sedation during monitoring, n (%) | 48 (54%) | 23 (54%) | 25 (56%) | 0.12 |
| None | 40 (46%) | 20 (46%) | 20 (44%) | |
| Propofol | 8 (9%) | 2 (5%) | 6 (13%) | |
| Fentanyl | 32 (36%) | 15 (35%) | 17 (38%) | |
| Dexmedetomidine | 5 (5%) | 5 (12%) | 0 (0%) | |
| Pentobarbital | 3 (3%) | 1 (2%) | 2 (4.4%) | |
| Brain herniation during monitoring, n (%) | 23 (26%) | 4 (9.3%) | 19 (42%) | <0.001 |
| Midline shift at septum, mm | 0 [0 – 4] | 0 [0 – 3] | 1 [0 – 6] | 0.08 |
| Hemoglobin on admission, g/dL | 12.2 ± 2.6 | 12.2 ± 2.7 | 12.3 ± 2.6 | 0.89 |
| Hemoglobin during monitoring, g/dL | 10.1 ± 1.9 | 10.0 ± 1.6 | 10.2 ± 2.1 | 0.59 |
| Glasgow coma scale score (median, IQR) | 6 [3 – 7] | 5 [3 – 7] | 7 [5 – 8] | 0.004 |
| Admission mRS | 0 [0 – 1] | 0 [0 – 2] | 0 [0 – 1] | 0.97 |
| MAP during monitoring (mean ± SD), mmHg | 90.1 ± 10.5 | 90.1 ± 10.4 | 90.4 ± 11.0 | 0.91 |
| pCO2 during monitoring (mean ± SD), mmHg | 38.3 ± 7.7 | 38.2 ± 7.2 | 38.3 ± 8.2 | 0.96 |
| Average rSO2 (median, IQR) | 60 [51 – 70] | 59 [51 – 70] | 61 [50 – 70] | 0.94 |
| Median COx (median, IQR) | 0.06 [0.02 – 0.14] | 0.04 [−0.01 – 0.11] | 0.08 [0.04 – 0.16] | 0.012* |
| Duratio8n of monitoring (hours) | 38.3 ± 24.2 | 38.4 ± 26.9 | 38.2 ± 21.7 | 0.97 |
Abbreviations: F: female, M: male, SD: standard deviation, IQR: interquartile range, pCO2: arterial pressure of carbon dioxide, ICP: intracranial pressure, MAP: mean arterial pressure, rSO2: regional cerebral oxygen saturation.
across all categories
Autoregulation index cut-offs and prediction of short and long-term functional outcome
The median COx of non-survivors at hospital discharge was higher (0.082, IQR 0.045–0.160) than that of survivors (0.042, IQR −0.005 to 0.110; P=0.012). Survival was significantly associated with lower values of COx (P=0.012). At 6 months, median COx was also higher in non-survivors (0.075, IQR 0.27–0.158) compared to survivors (0.029, IQR −0.015 to 0.077; P=0.02). Furthermore, patients with severe disability at 6 months had significantly higher median COx (0.072 [IQR 0.019–0.154] vs. 0.018 [IQR −0.035 to 0.066]; P=0.04). rSO2 was not associated with mortality either at discharge (P=0.94) or at 6 months (P=0.86). Figure 1 shows values of COx for each mRS value at hospital discharge.
Figure 1.
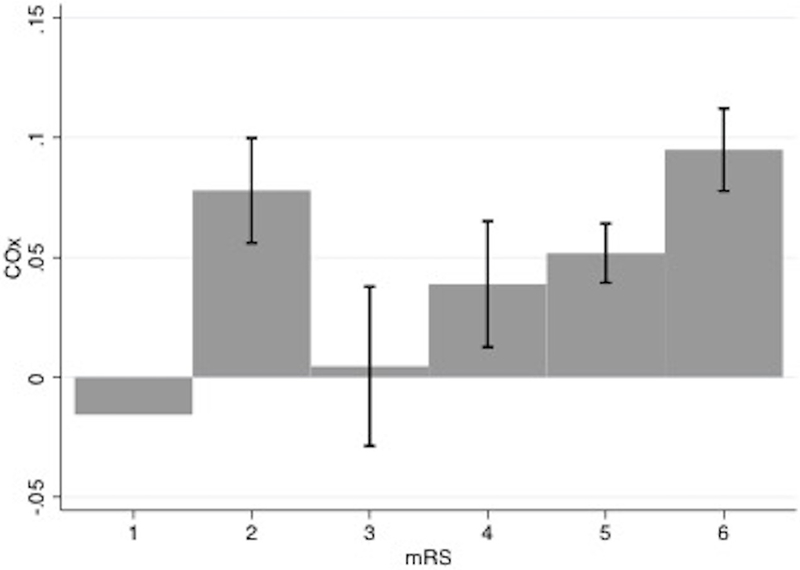
Mean COx with standard error values distributed by modified Rankin scale (mRS) at hospital discharge. Numbers of patients in each mRS group was: 1 (2 patients), 2 (3 patients), 3 (4 patients), 4 (16 patients), 5 (18 patients), 6 (45 patients).
We found that COx of 0.05 was the optimal cutoff for predicting both short and long-term mortality and severe disability. In the multivariable logistic regression model, after adjusting for age and GCS at the start of monitoring, we found that average COx ≥0.05 was significantly associated with mortality at hospital discharge (adjusted Odds Ratio [aOR] =2.9, 95% CI=1.15–7.33; P=0.024), at 3 months (aOR=4.72, 95% CI=1.59–14.1; P=0.005) and at 6 months (aOR=4.4, 95% CI=1.41–13.7; P=0.01) (Table 3). In the multivariable model adjusting for the same confounders, Cox ≥0.05 was significantly associated with mRS ≥4 at 6 months (aOR=4.4, 95% CI=1.07–17.8; P=0.04), but not at hospital discharge (aOR 2.9, 95% CI 0.68– 12.8; P=0.145) nor at 3 months (aOR=2.64, 95% CI=0.67–10.5; P=0.167) (Table 4). Areas under the ROC curve for predicting mortality and severe disability at 6 months were 0.783 and 0.825, respectively (Figure 2). COx accuracy to predict short and long-term mortality and long-term poor functional outcome in this population of acutely comatose adults showed an intermediate PPV (59–68%) and NPV (55–69%).
Table 3.
Logistic regression multivariable model for prediction of mortality
| Variable | aOR | 95% CI | P |
|---|---|---|---|
| At hospital discharge (n=88) | |||
| COx > 0.05 | 2.90 | 1.15 – 7.33 | 0.024 |
| GCS at start of monitoring | 0.69 | 0.54 – 0.89 | 0.004 |
| Age | 1.01 | 0.98 – 1.05 | 0.471 |
| At 3 months (n=74) | |||
| COx > 0.05 | 4.72 | 1.59 – 14.1 | 0.005 |
| GCS at start of monitoring | 0.64 | 0.47 – 0.87 | 0.004 |
| Age | 1.01 | 0.97 – 1.05 | 0.644 |
| At 6 months (n=73) | |||
| COx > 0.05 | 4.40 | 1.41 – 13.7 | 0.010 |
| GCS at start of monitoring | 0.63 | 0.46 – 0.88 | 0.006 |
| Age | 0.98 | 0.94 – 1.02 | 0.381 |
Abbreviations: aOR: adjusted odds ratio; CI: confidence interval; GCS: Glasgow Outcome Scale.
Table 4.
Logistic regression multivariable model to predict poor outcome (mRS ≥4)
| Variable | aOR | 95% CI | P |
|---|---|---|---|
| At hospital discharge (n=88) | |||
| COx > 0.05 | 2.96 | 0.68 – 12.8 | 0.145 |
| GCS at start of monitoring | 1.05 | 0.72 – 1.52 | 0.796 |
| Age | 1.00 | 0.95 – 1.05 | 0.901 |
| At 3 months (n=74) | |||
| COx > 0.05 | 2.64 | 0.67 – 10.5 | 0.167 |
| GCS at start of monitoring | 0.62 | 0.39 – 0.97 | 0.038 |
| Age | 1.02 | 0.66 – 10.5 | 0.405 |
| At 6 months | |||
| COx > 0.05 | 4.38 | 1.08 – 17.8 | 0.039 |
| GCS at start of monitoring | 0.54 | 0.33 – 0.89 | 0.016 |
| Age | 0.97 | 0.93 – 1.03 | 0.359 |
Abbreviations: mRS: modified Rankin Score; aOR: adjusted odds ratio; CI: confidence interval
Figure 2.
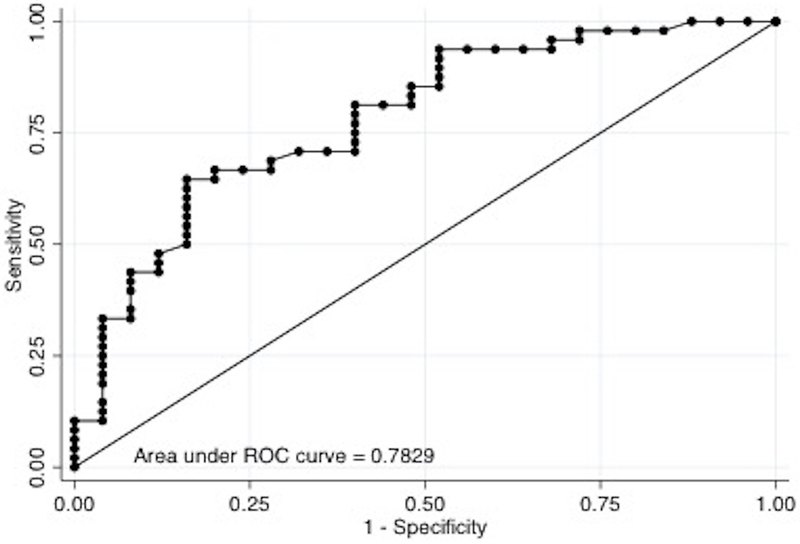
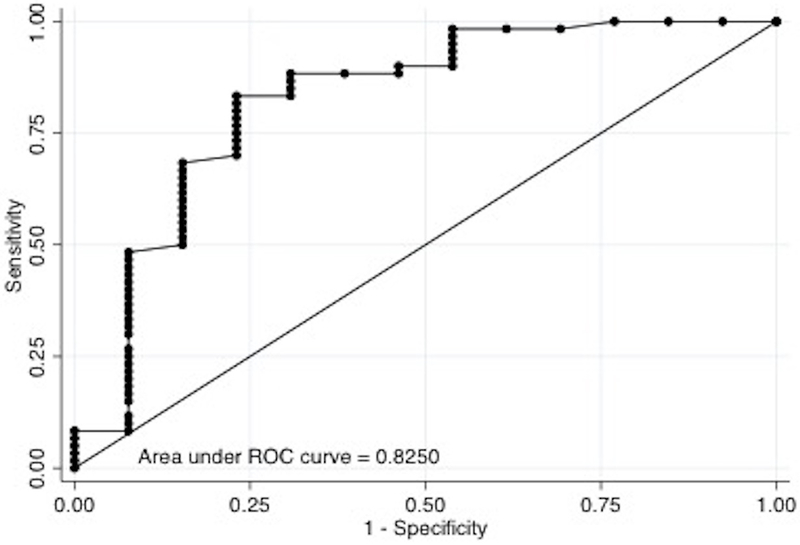
(A) ROC curve for mortality at 6 months (n=73).
(B) ROC curve for poor outcome (mRS≥4) at 6 months (n=73).
Impact of COx variation on different coma etiologies
We studied the variation of COx in the different subgroups of acute coma. Patients with ventriculitis and ischemic stroke had the highest median COx of 0.16 and 0.12 [IQR 0.01 – 0.18], respectively. This was followed by patients with intracerebral hemorrhage with COx 0.07 [IQR 0.03 – 0.15], aneurysmal subarachnoid hemorrhage with COx 0.06 [IQR 0.03 – 0.10] and traumatic brain injury with COx 0.06 [IQR 0.02 – 0.18]. The group with status epilepticus had the lowest COx - 0.00 [IQR −0.07 – 0.04], suggesting the most intact autoregulation.
Impact of MAP variation on cerebral autoregulation measurements
Differences in the strength of the correlation between NIRS oximetry and MAP could be secondary to a greater spread of MAP in non-survivors and not necessarily due to inherent differences in vascular reactivity, especially if the variation in MAP is small. However, we found that the SD of MAP in those that survived to hospital discharge averaged 7.15 mmHg, which was not significantly different from the average SD of 9.04 mmHg in non-survivors (P = 0.12). Likewise, the SD of MAP during the monitoring period of those that survived 6 months averaged 7.12 mmHg, which was not significantly different from that of non-survivors (8.95 mmHg; P = 0.13). Thus, the 2 mmHg difference in the MAP SD averaged across patients was unlikely to account for the differences in the COx between groups.
DISCUSSION
In this study we investigated the accuracy for outcome prediction of bedside continuous NIRS-derived CA monitoring in comatose patients with acute brain injury. We demonstrated that average COx values ≥0.05 in acutely comatose patients are an independent predictor for short and long-term mortality, and long-term severe disability. These findings support the use of bedside NIRS-derived COx as a potentially useful method of neuromonitoring to help prognosticate functional outcome after acute coma.
Previous studies have demonstrated the predictive value of NIRS-derived CA indices in prognostication among patients with traumatic brain injury, sepsis, hypoxic ischemic encephalopathy, or patients undergoing cardiac surgery.18, 19, 22, 27–36 Our study supports the applicability of this CA index to a broader population of neuro-critically ill patients with different coma etiologies, with a threshold value of COx apparently agnostic to the underlying diagnosis. This may be plausible as COx depends on the proportion of oxygenated hemoglobin and MAP, both important regulators of brain function. However, a single index of CA or different indices may perform differently in different disease conditions. Schmidt et al. studied 41 patients with both traumatic and non-traumatic causes of brain injury and found that, although both PRx and Mx were significantly associated with 3-month clinical outcome, PRx performed better in TBI patients while Mx had stronger correlation with outcomes in non-traumatic patients; prognostic values of indexes were also affected by conditions such as diabetes and hypocapnia.17 Therefore, further investigation is required to understand how outcome predictive values of COx may depend on patient characteristics. The strength of the current study is that of opening a new opportunity for the applicability of NIRS-derived CA measurements in comatose populations regardless of etiology, to assist in the prediction of short and long-term functional outcomes. In the near future, we anticipate that more innovative optical technologies, such as diffuse correlation spectroscopy, will permit measurement of actual CBF rather than just of rSO2. This new technology allows CBF measurement by quantifying temporal fluctuations of NIR light caused by the dynamic scattering of the moving red blood cells.37
COx thresholds from NIRS-based technology have previously been described in a cardiopulmonary bypass (CPB) patient population. Brady et al. reported a cut-off of 0.36 as the threshold of impaired autoregulation using the INVOS pediatric cerebral oximeter probe (Somanetics) in piglets anesthetized with inhaled isoflurane and nitric oxide and fentanyl.10 In patients with aneurysmal SAH using a NIRO 300 [Hamamatsu Photonics, Hamamatsu city, Japan],38 the threshold for predicting delayed cerebral ischemia was found to be 0.1 for the tissue oximetry index (TOx).24 These two NIRS devices use different measurement techniques for measuring regional tissue oxygen saturation and different algorithms for rSO2 calculation.39 Ours is the first study of NIRS derived CA index thresholds using the INVOS device in comatose patients, and identified an even lower cut-off of 0.05, which requires further explanation. Donegan et al.40 investigated CA in adult sheep with induced coma and found that the CA plateau extended over a wider CPP range compared to awake sheep; consequently the CA indices were more negative and closer to zero. By lowering the brain’s oxygen demand, the cerebral vessels constrict at normal MAP and thereby have a greater vasodilatory reserve to handle a low MAP, resulting in a plateau for CA which extends over a wider CPP range. Another factor impacting the plateau is the type of brain injury. Patients with severe brain injury and poor brain compliance may have a smaller plateau compared to those without intrinsic brain injury on imaging, such as patients with well treated status epilepticus. This can be seen in table 2 which shows that patients with status epilepticus in our study were always autoregulated, with a median COx of 0.00. Compared to studies of patients undergoing CPB, we would not expect comatose patients in the neurocritical care unit to have the same COx threshold for prognostication. The physiology of the former is impacted by exposure to inhalational anesthesia which vasodilates the cerebral blood vessels; however this effect may be counteracted by the vasoconstrictive effects of hypothermia use during CPB. Finally, Moerman et al. assessed CA patterns with NIRS during pharmacologically induced pressure changes in patients undergoing cardiac surgery.41 In the group with higher MAP, COx was significantly more negative and closer to zero. In our population, 23.8% of patients had brain herniation. These patients required a higher CPP to counteract elevated ICP, and indeed the average MAP was higher in this study (91.2 ± 10.8 mmHg) compared to other populations, for example 72.2 ± 11.1 mmHg in Brady’s study.34. When MAP increases and CA is intact, arteries and arterioles increase their tone to prevent distension from increases in transmural pressure. If the increase in tone is sufficient to decrease arteriolar diameter, vascular resistance increases. This limits the increase in CBF associated with an increase in CPP. If the increase in tone is very large, the decrease in diameter may be so severe as to actually decrease CBF in the face of increased CPP. This “super-autoregulation” response could yield a negative CA index in patients with severe brain injury.40
Table 2.
Different acute coma etiologies and their respective median COx and interquartile range.
| Coma etiology (n) | Median COx (median, IQR) |
|---|---|
| Ischemic stroke (10) | 0.12 [0.01 – 0.18] |
| Intracerebral hemorrhage (28) | 0.07 [0.03 – 0.15] |
| Aneurysmal subarachnoid hemorrhage (24) | 0.06 [0.03 – 0.10] |
| Traumatic brain injury (11) | 0.06 [0.02 – 0.18] |
| Status epilepticus (7) | 0.00 [−0.07 – 0.04] |
| Meningitis (1) | 0.05 |
| Hypoxic ischemic encephalopathy (2) | 0.02 [0.00 – 0.05] |
| Ventriculitis (1) | 0.16 |
Abbreviations: COx, cerebral oximetry index; IQR: interquartile range.
COx accuracy to predict short and long-term mortality and long-term poor functional outcome in this population of acutely comatose adults showed an intermediate PPV (59–68%) and NPV (55–69%). These findings may be related to the averaging of COx over the duration of monitoring. Patients may have started with intact autoregulation which later worsened, and the average levels of COx diluted the prediction accuracy. Alternatively, the clinical condition could have deteriorated after the completion of the 3 days of monitoring, and subsequent changes in COx were not captured.
In this prospective cohort, rSO2 was not associated with mortality or severe disability. This is in line with prior literature showing that absolute rSO2 values are less accurate, whereas changes in rSO2, which are used to derive COx, are more valid.42 Conversely, some studies have shown a significant association between these measurements and mortality in TBI, risk of extubation failure in pediatric patients undergoing cardiac surgery, delayed cerebral ischemia in subarachnoid hemorrhage,20 and neurologic outcomes post-cardiac arrest.19, 43–45 Vilke et al. showed that rSO2 provides a stronger predictive value for hospital mortality in traumatic brain injury than traditional parameters such as admission GCS.19 However these authors did not perform multivariable analysis to adjust for possible confounders and their study had a small sample size. The predictive value of COx has been demonstrated in patients undergoing abdominal and cardiac surgery for postoperative cognitive dysfunction and acute kidney injury.22, 34, 35, 46
Given the low threshold of 0.05 in the heterogeneous cohort in our study, further investigation of COx in more homogeneous disease populations will be needed before COx assessment could become a clinically useful guide for predicting patient outcomes or counseling families of unconscious patients. Our data do, however, support the need to assess CA and calculate optimal MAP at the bedside,9 with the goal of optimizing CPP in these critically ill patients using non-invasive devices.23
This study has several limitations, notably related to the relatively small sample size and inherent limitations of the methodology. These may explain the significant overlap between COx distributions in patients with poor and good outcomes and therefore the relatively low predictive values. Moreover, the heterogeneity of the study pathology, including parenchymal hematomas, cerebral edema and subarachnoid blood, may affect interpretability of the results. However, our aim was to assess the predictive value for a general population of comatose patients. Future analysis of COx by coma etiology, lesion location and underlying pathophysiology (vasospasm, edema, ischemia, etc.) will require larger populations. Secondly, the use of rSO2 as a surrogate of CBF for CA monitoring is based on assumptions that other determinants of rSO2 (i.e. tissue oxygen diffusivity, CMRO2, temperature) are relatively stable over the low frequencies of vasoreactivity that mediate CA. Other physiologic variables that alter brain oxygen supply and demand may also affect rSO2. These include cardiac output, pulmonary function, systemic hypoxemia, acid-base status and PaCO2, which affect not only the hemoglobin saturation for a given PO2 but also CBF.47 Cerebral oximetry monitors a mixed vascular bed dominated by gas-exchanging vessels, especially venules.48 The arterial/venous ratio is assumed to be 16:84, but this may be different in the injured brain. The variable duration of monitoring per patient (from 1 hour through 3 days) could also affect the results; however, we found no significant difference in duration of monitoring between survivors and non-survivors (table 1). NIRS measurements are also contaminated by the extracranial vasculature. In one study, the change in rSO2 due to extracranial contamination was be up to 16.6±9.6%.49 Anatomically, our study was not powered to account for differences in autoregulatory measurements between patients with frontal lesions and those without.
CONCLUSION
Average COx ≥ 0.05 is independently associated with short and long-term mortality and long-term severe disability in acutely comatose adults with neurological injury. Further studies with larger populations should be performed with this technology to refine recommendations for disease-specific clinical use.
Acknowledgments
Sources funding: Dr. Hogue is the PI on an NIH-sponsored clinical study (R01 HL 92259). Dr. Rivera Lara is the PI on an American Academy of Neurology/American Brain Foundation and grant from Medtronic/Covidien, Dublin, IR.
Disclosures: Dr. Hogue receives research funding from Medtronic/Covidien, Dublin, IR, and he serves as a consultant to Medtronic/Covidien and Ornim Medical, Inc., Foxborough, MA.
REFERENCES
- 1.Hemphill JC. It’s getting better all the time? Using secular trends to understand the impact of neurocritical care. Intensive care medicine. 2013;39:1489–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim N, Krasner A, Kosinski C, Wininger M, Qadri M, Kappus Z, et al. Trending autoregulatory indices during treatment for traumatic brain injury. Journal of clinical monitoring and computing. 2015 [DOI] [PubMed] [Google Scholar]
- 3.Budohoski KP, Czosnyka M, Smielewski P, Varsos GV, Kasprowicz M, Brady KM, et al. Cerebral autoregulation after subarachnoid hemorrhage: Comparison of three methods. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang EW, Kasprowicz M, Smielewski P, Santos E, Pickard J, Czosnyka M. Short pressure reactivity index versus long pressure reactivity index in the management of traumatic brain injury. Journal of neurosurgery. 2015;122:588–594 [DOI] [PubMed] [Google Scholar]
- 5.Lang EW, Kasprowicz M, Smielewski P, Santos E, Pickard J, Czosnyka M. Outcome, pressure reactivity and optimal cerebral perfusion pressure calculation in traumatic brain injury: A comparison of two variants. Acta neurochirurgica. Supplement. 2016;122:221–223 [DOI] [PubMed] [Google Scholar]
- 6.Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Critical care medicine. 2012;40:2456–2463 [DOI] [PubMed] [Google Scholar]
- 7.Green MS, Sehgal S, Tariq R. Near-infrared spectroscopy: The new must have tool in the intensive care unit? Seminars in cardiothoracic and vascular anesthesia. 2016 [DOI] [PubMed] [Google Scholar]
- 8.Czosnyka M, Miller C. Monitoring of cerebral autoregulation. Neurocritical care. 2014;21 Suppl 2:S95–102 [DOI] [PubMed] [Google Scholar]
- 9.Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocritical care. 2009;10:122–128 [DOI] [PubMed] [Google Scholar]
- 10.Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke; a journal of cerebral circulation. 2007;38:2818–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: A systematic review. Telemedicine journal and e-health : the official journal of the American Telemedicine Association. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budohoski KP, Zweifel C, Kasprowicz M, Sorrentino E, Diedler J, Brady KM, et al. What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. British journal of anaesthesia. 2012;108:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Highton D, Ghosh A, Tachtsidis I, Panovska-Griffiths J, Elwell CE, Smith M. Monitoring cerebral autoregulation after brain injury: Multimodal assessment of cerebral slow-wave oscillations using near-infrared spectroscopy. Anesthesia and analgesia. 2015;121:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke; a journal of cerebral circulation. 2009;40:1820–1826 [DOI] [PubMed] [Google Scholar]
- 15.Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, Healy R, Radziq B, Palmisano C, et al. Validation of near-infrared spectroscopy for monitoring cerebral autoregulation in comatose patients. Neurocritical care. 2017;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono M, Zheng Y, Joshi B, Sigl JC, Hogue CW. Validation of a stand-alone near-infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery. Anesthesia and analgesia. 2013;116:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt B, Reinhard M, Lezaic V, McLeod DD, Weinhold M, Mattes H, et al. Autoregulation monitoring and outcome prediction in neurocritical care patients: Does one index fit all? Journal of clinical monitoring and computing. 2016;30:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies DJ, Su Z, Clancy MT, Lucas SJ, Dehghani H, Logan A, et al. Near-infrared spectroscopy in the monitoring of adult traumatic brain injury: A review. Journal of neurotrauma. 2015;32:933–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilke A, Bilskiene D, Saferis V, Gedminas M, Bieliauskaite D, Tamasauskas A, et al. Predictive value of early near-infrared spectroscopy monitoring of patients with traumatic brain injury. Medicina (Kaunas, Lithuania). 2014;50:263–268 [DOI] [PubMed] [Google Scholar]
- 20.Yousef KM, Balzer JR, Crago EA, Poloyac SM, Sherwood PR. Transcranial regional cerebral oxygen desaturation predicts delayed cerebral ischaemia and poor outcomes after subarachnoid haemorrhage: A correlational study. Intensive & critical care nursing. 2014;30:346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, et al. Simplified modified rankin scale questionnaire: Reproducibility over the telephone and validation with quality of life. Stroke; a journal of cerebral circulation. 2011;42:2276–2279 [DOI] [PubMed] [Google Scholar]
- 22.Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Critical care medicine. 2013;41:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, Healy RJ, Ziai W, Mirski MA. Cerebral autoregulation-oriented therapy at the bedside: A comprehensive review. Anesthesiology. 2017 [DOI] [PubMed] [Google Scholar]
- 24.Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: A prospective observational study. Stroke; a journal of cerebral circulation. 2012;43:3230–3237 [DOI] [PubMed] [Google Scholar]
- 25.Weerakkody RA, Czosnyka M, Zweifel C, Castellani G, Smielewski P, Keong N, et al. Slow vasogenic fluctuations of intracranial pressure and cerebral near infrared spectroscopy--an observational study. Acta neurochirurgica. 2010;152:1763–1769 [DOI] [PubMed] [Google Scholar]
- 26.Sorrentino E, Budohoski KP, Kasprowicz M, Smielewski P, Matta B, Pickard JD, et al. Critical thresholds for transcranial doppler indices of cerebral autoregulation in traumatic brain injury. Neurocritical care. 2011;14:188–193 [DOI] [PubMed] [Google Scholar]
- 27.Kessel B, Alfici R, Korin A, Olsha O, Dudkiewicz M, Oren M. Real time cerebral perfusion monitoring in acute trauma patients: A preliminary study. ANZ journal of surgery. 2016 [DOI] [PubMed] [Google Scholar]
- 28.Massaro AN, Govindan RB, Vezina G, Chang T, Andescavage NN, Wang Y, et al. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Journal of neurophysiology. 2015;114:818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashida K, Nishiyama K, Suzuki M, Abe T, Orita T, Ito N, et al. Estimated cerebral oxyhemoglobin as a useful indicator of neuroprotection in patients with post-cardiac arrest syndrome: A prospective, multicenter observational study. Critical care (London, England). 2014;18:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito N, Nanto S, Nagao K, Hatanaka T, Kai T. Regional cerebral oxygen saturation predicts poor neurological outcome in patients with out-of-hospital cardiac arrest. Resuscitation. 2010;81:1736–1737 [DOI] [PubMed] [Google Scholar]
- 31.Burton VJ, Gerner G, Cristofalo E, Chung SE, Jennings JM, Parkinson C, et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC neurology. 2015;15:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: A review of transcranial doppler studies. Stroke; a journal of cerebral circulation. 2010;41:2697–2704 [DOI] [PubMed] [Google Scholar]
- 33.Berg RM, Plovsing RR. Near-infrared spectroscopy versus transcranial doppler ultrasound for assessing dynamic cerebral autoregulation by transfer function analysis in sepsis. Scandinavian journal of clinical and laboratory investigation. 2016;76:88–91 [DOI] [PubMed] [Google Scholar]
- 34.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke; a journal of cerebral circulation. 2010;41:1951–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hori D, Hogue C, Adachi H, Max L, Price J, Sciortino C, et al. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interactive cardiovascular and thoracic surgery. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Ji B, Yang B, Liu G, Miao N, Yang J, et al. Real-time continuous neuromonitoring combines transcranial cerebral doppler with near-infrared spectroscopy cerebral oxygen saturation during total aortic arch replacement procedure: A pilot study. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2012;58:122–126 [DOI] [PubMed] [Google Scholar]
- 37.Buckley EM, Parthasarathy AB, Grant PE, Yodh AG, Franceschini MA. Diffuse correlation spectroscopy for measurement of cerebral blood flow: Future prospects. Neurophotonics. 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niro-200nx near infrared oxygenation monitor.
- 39.Yoshitani K, Kawaguchi M, Tatsumi K, Kitaguchi K, Furuya H. A comparison of the invos 4100 and the niro 300 near-infrared spectrophotometers. Anesthesia and analgesia. 2002;94:586–590; table of contents [DOI] [PubMed] [Google Scholar]
- 40.Donegan JH, Traystman RJ, Koehler RC, Jones MD, Jr., Rogers MC. Cerebrovascular hypoxic and autoregulatory responses during reduced brain metabolism. The American journal of physiology. 1985;249:H421–429 [DOI] [PubMed] [Google Scholar]
- 41.Moerman AT, Vanbiervliet VM, Van Wesemael A, Bouchez SM, Wouters PF, De Hert SG. Assessment of cerebral autoregulation patterns with near-infrared spectroscopy during pharmacological-induced pressure changes. Anesthesiology. 2015;123:327–335 [DOI] [PubMed] [Google Scholar]
- 42.Rogers CA, Stoica S, Ellis L, Stokes EA, Wordsworth S, Dabner L, et al. Randomized trial of near-infrared spectroscopy for personalized optimization of cerebral tissue oxygenation during cardiac surgery. British journal of anaesthesia. 2017;119:384–393 [DOI] [PubMed] [Google Scholar]
- 43.Foster CB, Spaeder MC, McCarter RJ, Cheng YI, Berger JT. The use of near-infrared spectroscopy during an extubation readiness trial as a predictor of extubation outcome. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:587–592 [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama K, Ito N, Orita T, Hayashida K, Arimoto H, Beppu S, et al. Regional cerebral oxygen saturation monitoring for predicting interventional outcomes in patients following out-of-hospital cardiac arrest of presumed cardiac cause: A prospective, observational, multicentre study. Resuscitation. 2015;96:135–141 [DOI] [PubMed] [Google Scholar]
- 45.Zorrilla-Vaca A, Healy R, Grant MC, Joshi B, Rivera-Lara L, Brown C, et al. Intraoperative cerebral oximetry-based management for optimizing perioperative outcomes: A meta-analysis of randomized controlled trials. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2018;65:529–542 [DOI] [PubMed] [Google Scholar]
- 46.Sorensen H, Grocott HP, Secher NH. Near infrared spectroscopy for frontal lobe oxygenation during non-vascular abdominal surgery. Clinical physiology and functional imaging. 2015 [DOI] [PubMed] [Google Scholar]
- 47.Vretzakis G, Georgopoulou S, Stamoulis K, Stamatiou G, Tsakiridis K, Zarogoulidis P, et al. Cerebral oximetry in cardiac anesthesia. Journal of thoracic disease. 2014;6 Suppl 1:S60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93:947–953 [DOI] [PubMed] [Google Scholar]
- 49.Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: A comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116:834–840 [DOI] [PubMed] [Google Scholar]


