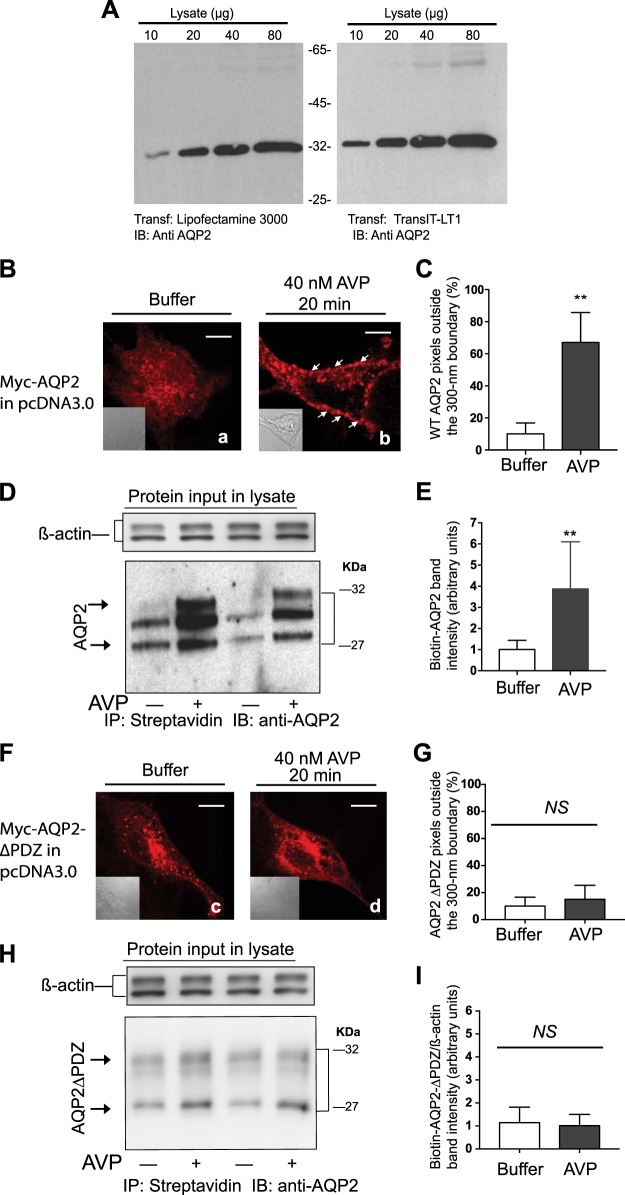
Fig. 1.
Role of the type-1 PDZ domain of aquaporin-2 (AQP2) in its translocation from storage vesicles to the plasma membrane of LLC-PK1 cells. A: effect of two transfection reagents on c-Myc-AQP2 expression in LLC-PK1 cells. B: distribution of wild-type (WT) c-Myc-AQP2 in LLC-PK1 cells that were exposed either to buffer (a) or 40 nM arginine-vasopressin (AVP; b) was determined by confocal microscopy. Nomarski images (inset) were acquired at the same magnification. Scale bars = 5 μm. C: averages ± SD of AQP2 pixels outside a 300-nm partition derived from 30 control (open bars) or AVP-exposed (closed bars) images from n = 3 experiments. Unpaired t-tests between the percentile of pixels outside the 300-nm partition in control versus AVP-treated cells are expressed as no significant difference (NS) or *P < 0.05, **P < 0.01, and **P < 0.001, respectively. D: total surface biotinylated WT AQP2 levels (arrows) in control versus AVP-treated LLC-PK1 cells. E: combined densitometry of biotinylated AQP2 in control versus AVP-treated cells that were corrected for β-actin levels in input lysates from five separate experiments (mean ± SD) were compared by Student’s t-test. F and H: distribution of AQP2∆PDZ in LLC-PK1 cells in response to buffer (c) or AVP (d) was determined either by confocal microscopy (F) or cell surface biotinylation (H), as described above. G and I: percentile of AQP2∆PDZ pixels from 30 images (n = 3) residing outside the 300-nm partition (G) and combined densitometry of the biotinylated samples (I; n = 5) are shown as means ± SD. Statistical comparisons were conducted as described in C and E. IB, immunoblot; IP, immunoprecipitation.