Abstract
Traumatic brain injury (TBI) causes a variety of neuropathological manifestations including cognitive, emotional, physiological and psychological deficits. Physical exercise is known to ameliorate neurological impairments induced by various brain injuries. We investigated the effects of treadmill exercise on memory impairments due to TBI in relation to dopamine and D2 dopamine receptor. TBI was induced with an electromagnetic-controlled cortical impact device. The rats in the exercise groups were scheduled to run on a treadmill for 30 min once a day for 28 days after TBI induction. Then, step-down avoidance task, radial 8-arm maze test, immunohistochemistry for tyrosine hydroxylase (TH), and western blot for D2 dopamine receptor were performed. TBI impaired short-term and spatial learning memories. TBI decreased TH expressions in the prefrontal cortex (PFC), striatum, hippocampus dentate gyrus, and substantia nigra (SN). By contrast, the expressions of D2 dopamine receptor in the PFC, striatum, hippocampus, and SN were increased by TBI. Treadmill exercise alleviated the impairments of short-term and spatial learning memories observed in TBI rats. TH expression was decreased and D2 dopamine receptor expression was increased in TBI rats. Treadmill exercise enhanced TH expression and suppressed D2 dopamine receptor expression in TBI rats. TBI deteriorated short-term and spatial learning memories, in contrast, treadmill exercise alleviated the TBI-induced memory impairments by up-regulating dopamine level and down-regulating D2 dopamine receptor expression.
Keywords: Brain injuries, Exercise, Memory, Dopamine, Dopamine D2 receptor
INTRODUCTION
Traumatic brain injury (TBI) is an important cause of death and disability in developed countries. TBI causes a variety of neuropathological manifestations including cognitive, emotional, physiological and psychological deficits (Rockswold et al., 2010). Due to the complexity of injury and the variety of patient presentations, definitive therapeutic strategy for TBI has not been established.
Anatomical and functional studies suggest that the dopamine system may be vulnerable to TBI, and previous studies have shown that dopamine is suppressed after TBI (Huang et al., 2014; Massucci et al., 2004). Dopamine uses a unique signaling system in the central nerve system and dopamine acts as neurotransmitter and neuromodulator. Dopaminergic neurons originate from the substantia nigra (SN) and are projected to cerebral forebrain structures, including hippocampus, prefrontal cortex (PFC), and striatum (Crosson and Halland, 2003). Striatum is an important component of anatomic networks that support functions associated with dorsolateral PFC. In addition, striatum helps to mediate human cognition (Crosson and Halland, 2003; Wagner et al., 2014). Dopamine receptors are classified as subtypes of D1–D5 dopamine receptors based on receptor structure, ligand, and effector (Beaulieu and Gainetdinov, 2011; Zhang and Jiang, 2015). One of these subtypes, the D2 dopamine receptor is included in the family of G protein-coupled receptor. Activation of the D2 dopamine receptor inhibits synaptogenesis of dopamine-releasing neurons through translational regulation of protein synthesis necessary for synapse formation (Granado et al., 2008). In particular, D2 dopamine receptor is associated with TBI in humans and animals (Wagner et al., 2014; Zhang and Jiang, 2015).
Physical exercise promotes recovery processes, nerve regeneration, and nerve survival to protect brain function from multiple types of insults (Griesbach, 2011). Physical exercise also alleviates post-TBI neuropathy by inhibiting cell death, suppressing inflammation, inhibiting oxidative stress, and improving neural restoration (Archer et al., 2012; Piao et al., 2013). Running exercise also promotes recovery from nigrostriatal dopamine injury (O’Dell et al., 2007). However, the effects of physical exercise on memory associated with dopamine and its receptor after TBI were not clearly reported.
In the present study, we investigated the effects of treadmill exercise on impairments of short-term and spatial learning memories due to TBI in relation to dopamine and D2 dopamine receptor. For this study, step-down avoidance task, radial 8-arm maze test, immunohistochemistry for tyrosine hydroxylase (TH), and western blot for D2 dopamine receptor were performed.
MATERIALS AND METHODS
Animals and treatments
Male Sprague-Dawley rats, weighing 90±5 g (4 weeks old), were used for the experiments. All experimental procedures were carried out in accordance with the Guidelines for the Care and Use of Animals approved by the National Institutes of Health Council for management and use of laboratory animals. The study was approved by the Institutional Care and Use Committee of Kyung Hee University (KHUASP[SE]-18-086). The rats were housed under controlled temperature (23°C±2°C) and lighting (08:00 a.m. to 20:00 p.m.) conditions with food and water available ad libitum. The animals were randomly divided into the following four groups (n=10 in each group): sham-operation group, sham-operation and treadmill exercise group, TBI-induced group, and TBI-induced and treadmill exercise group.
Induction of TBI
The TBI model used in the present study was based on a previous study (Ko et al., 2018). For the induction of TBI, the rats were anesthetized with Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France). A midline incision was made on the skin and underlying fascia. A circular craniotomy (5.0 mm) was performed using a Dremel motor tool and a specially designed drill bit that prevented damage to the meninges and cortex (2.4 mm lateral to the midline, and 4.2 mm posterior to the coronal suture). The contusion injury was created with an electromagnetic contusion device (Impact One, Stereotaxic Impactor; MyNeurolab, St. Louis, MO, USA) using a sterile stainless-steel impactor tip (3.0 mm diameter) that was activated at a velocity of 3.00 m/sec. The impactor tip was positioned above the cortex, and resulted in a 2.5 mm compression to the cortex.
Exercise protocol
Treadmill exercise was performed as previously described method. Rats in the exercise groups were scheduled to run on a motorized treadmill for 30 min once a day for 28 days, starting 21 days after TBI induction. The exercise load consisted of running at speeds of 2 m/min for the first 5 min, 5 m/min for the next 5 min, and then 8 m/min for the last 20 min, at an inclination of 0°. The rats in the sham-operation group and in TBI-induced group were left in the treadmill, without running for the same period as the exercise groups.
Step-down avoidance task
The latency of the step-down avoidance task was determined to evaluate short-term memory, as previously described method (Lee et al., 2018). Rats were trained in a step-down avoidance task 47 days after TBI induction (26 days after starting the treadmill exercise). Two hours after training, the latency (sec) of each animal was determined. The rats were placed 7×25-cm platform 2.5 cm high. The platform faced a 42×25-cm grid of parallel 0.1-cm caliber stainless-steel bars spaced 1 cm apart. In training sessions, the animals received 0.5-mA scramble foot shock for 2 sec immediately upon stepping down. The interval of rats stepping down and placing all four paws on the grid was defined as the latency. A latency over 300 sec was counted as 300 sec.
Radial 8-arm maze test
Spatial learning memory was determined using a radial 8-arm maze apparatus, as previously described method (Lee et al., 2018). The radial-arm maze apparatus consisted of a central octagonal plate (30 cm in diameter) and eight radiating arms (50 cm in length and 10 cm in width). The apparatus was placed 1 m above the floor. A small receptacle filled with water (3 cm in diameter and 1 cm in depth) was located at the end of the arms. The rats were trained 3 times before the spatial learning test. The rats were deprived of water for 24 hr and allowed to explore the water for 5 min. The test was conducted on the 48 days after TBI-induced operation (27 days after starting the treadmill exercise). The time spent in seeking water at the end of the arms was counted. The test was terminated when a rat found water in all eight arms or when >8 min elapsed. Re-entry into the previously visited arms was counted as an error. In addition, the number of correct choices before the first error was counted.
Tissue preparation
The animals were sacrificed immediately after determining the spatial learning memory in the radial 8-arm test. Tissue preparation was performed as previously described method (Ko et al., 2018). The rats were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories), transcardially perfused with 50 mM phosphate-buffered saline, and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (pH, 7.4). Brains were dissected, and storage overnight same fixative, then it was transferred to 30% sucrose for cryoprotection. For the immunohistochemistry, the slices were coronal sectioned at 40 μm thick using a cryostat (Leica, Nussloch, Germany). Ten slice sections on average containing PFC, striatum, hippocampus, and SN were collected from each rat.
Immunohistochemistry for TH
An immunohistochemistry was conducted to evaluate the TH expressions in the PFC, striatum, hippocampal dentate gyrus, and SN, as previously described method (Ko et al., 2013). Free-floating tissue sections were incubated overnight with mouse anti-TH antibodies (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:1,000, and the sections were then incubated for 1 hr with biotinylated anti-mouse for TH secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Next, the sections were incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 hr at room temperature. For staining, the sections were incubated in a reaction mixture consisting of 0.03% diaminobenzidine and 0.03% hydrogen peroxide for 5 min. The sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount (Fisher Scientific, New Jersey, NJ, USA).
Western blotting
The PFC, striatum, hippocampus, and SN tissues were dissected and collected, and then were immediately frozen at −70°C. Western blot analysis was performed as previously described method (Ko et al., 2013). The sample tissues were homogenized on ice, and lysed in a lysis buffer containing 50 mM HEPES (pH, 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1 mM EGTA, 1.5 mM MgCl2·6H2O, 1 mM sodium orthovanadate, and 100 mM sodium fluoride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein samples (30 μg) were separated on sodium dodecyl sulfate-polyacrylamide gel and transferred onto a nitrocellulose membrane. Rabbit GAPDH antibody (1:5,000; AbFrontier, Seoul, Korea) and mouse D2 dopamine receptor antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody for D2 dopamine receptor (1:2,000; Vector Laboratories) and anti-rabbit antibody for GAPDH (1:3,000; Vector Laboratories) were used as the secondary antibodies. The experiment was performed in normal lab conditions at room temperature except for membrane transfer. Membrane transfer was performed at 4°C with the cold pack and prechilled buffer. Band detection was performed using the enhanced chemiluminescence detection kit (Santa Cruz Biotechnology).
Data analysis
The number of TH-positive cells in the SN and hippocampal dentate gyurs was counted using a light microscope (Olympus, Tokyo, Japan). The area of the granular layer of the hippocampal dentate gyrus from each slice was measured by Image-Pro Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). The optical densities of TH-immunoreactive fibers in the PFC and striatum were measured in 100×100-μm square images using an image analyzer (Multiscan, Fullerton, CA, USA). To estimate the TH-staining densities, the optical densities were corrected for the nonspecific background density, which was measured in the completely denervated parts of the PFC and striatum. The optical densities of TH-positive fibers in the PFC and striatum were calculated as follows: the optical density in the lesion side/the optical density in the intact side. In order to compare relative expression of proteins, detected bands were calculated densitometrically using Molecular Analyst ver. 1.4.1 (Bio-Rad). Statistical analysis was performed using one-way analysis of variance followed by Duncan post hoc test, and the results are expressed as the mean±standard error of the mean. Significance was set as P<0.05.
RESULTS
Effects of treadmill exercise on the short-term memory
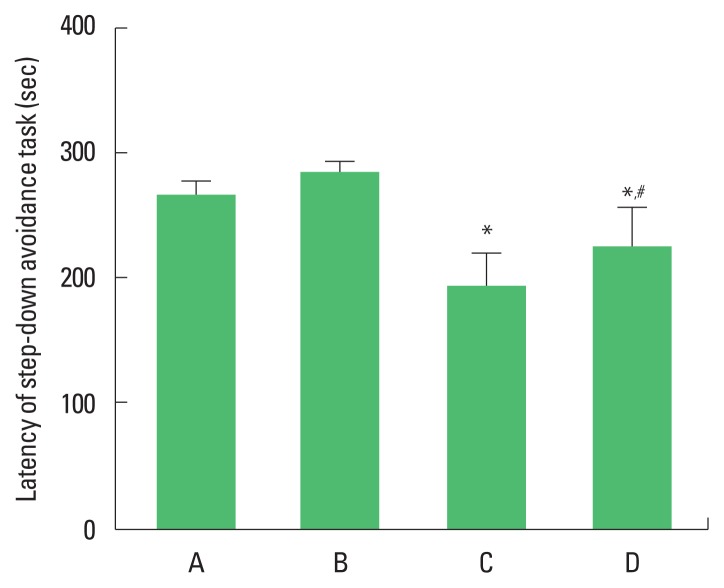
The latencies in the step-down avoidance task are presented in Fig. 1. The present results show that TBI caused deterioration of short-term memory (P<0.05) and that treadmill exercise alleviated the TBI-induced impairments of short-term memory (P<0.05).
Fig. 1.
Effects of treadmill exercise on latency in the step-down avoidance task. A, sham-operation group; B, sham-operation and treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the TBI-induced group.
Effects of treadmill exercise on the spatial learning memory
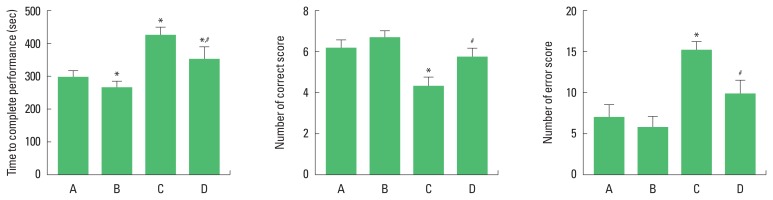
The time of successful performance, the number of the correct, and the number of error choices in the radial 8-arm maze test are presented in Fig. 2. The present results show that TBI-induced rats required more time for completing the task, made fewer correct choices (P<0.05), and had more errors compared to the control rats (P<0.05). In contrast, rats with TBI who performed treadmill exercise completed the test in less time (P<0.05), made more correct choices (P<0.05), and had fewer errors (P<0.05) compared to the TBI-induced rats.
Fig. 2.
Effects of treadmill exercise on performance time, correct number, and error score in the radial 8-arm maze test. A, sham-operation group; B, sham-operation and treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the TBI-induced group.
Effects of treadmill exercise on TH expressions
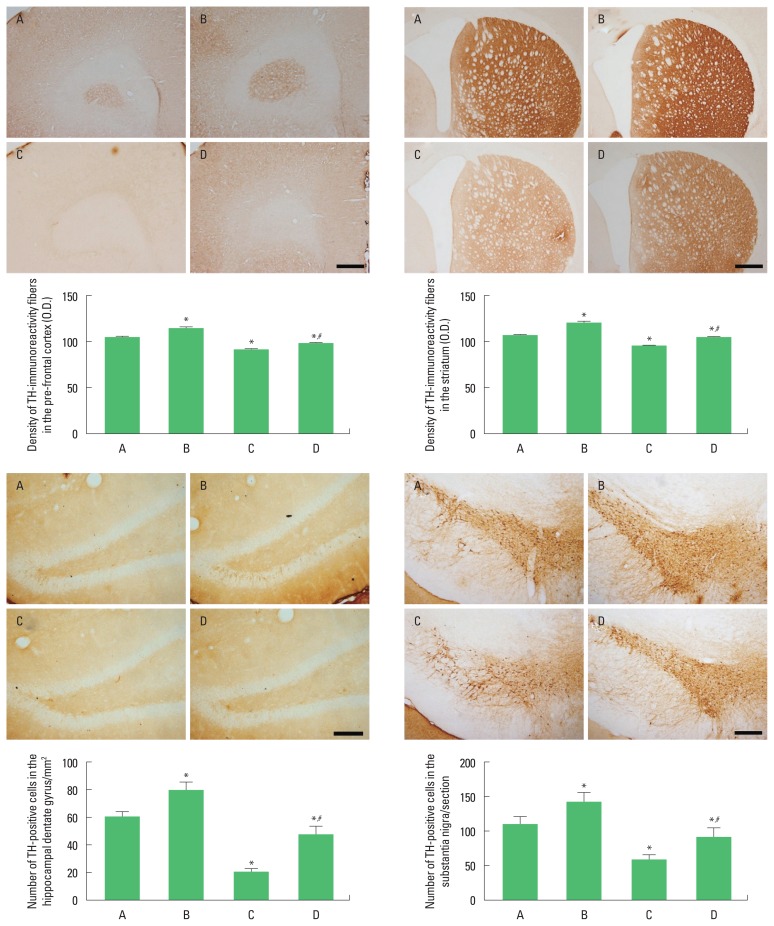
Photomicrographs of TH expressions in the PFC, striatum, hippocampal dentate gyrus, and SN are presented in Fig. 3. The present results show that induction of TBI enhanced TH expressions in the PFC, striatum, hippocampal dentate gyrus, and SN (P<0.05), whereas treadmill exercise suppressed TH expressions in the PFC, striatum, hippocampal dentate gyrus and SN of rats with TBI (P<0.05).
Fig. 3.
Effects of treadmill exercise on tyrosine hydroxylase (TH) expressions in the prefrontal cortex, striatum, hippocampal dentate gyrus, and substantia nigra. A, sham-operation group; B, sham-operation and treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group. The scale bar represents 150 μm. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the TBI-induced group.
Effects of treadmill exercise on D2 dopamine receptor expressions
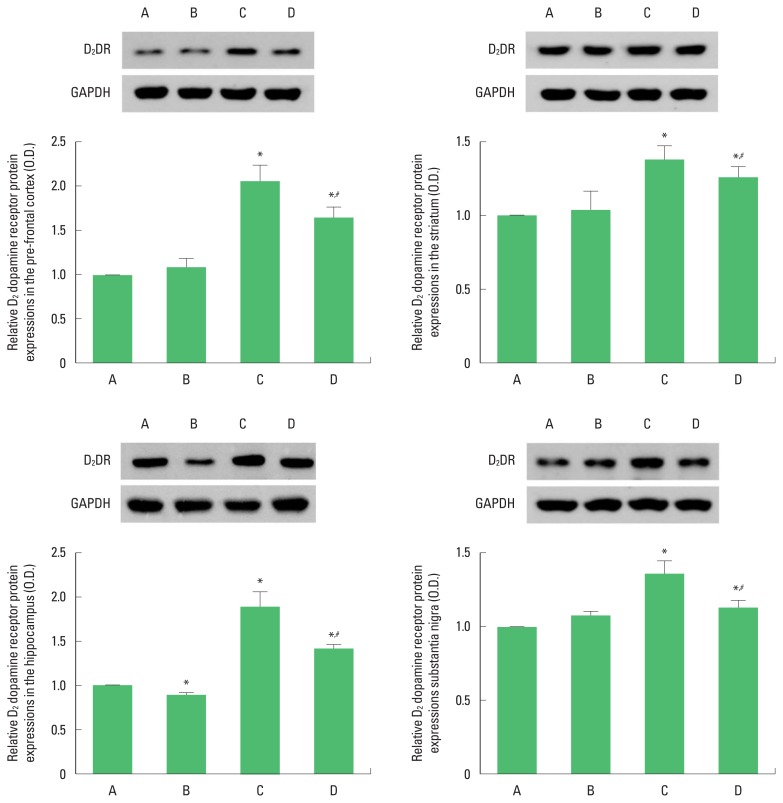
Photomicrographs of D2 dopamine receptor expressions in the PFC, striatum, hippocampal dentate gyrus, and SN are presented in Fig. 4. The present results show that induction of TBI enhanced D2 dopamine receptor expressions in the PFC, striatum, hippocampus, and SN (P<0.05), whereas treadmill exercise suppressed D2 dopamine receptor expressions in the PFC, striatum, hippocampal dentate gyrus and SN of rats with TBI (P<0.05).
Fig. 4.
Effects of treadmill exercise on D2 dopamine receptor (D2DR) expressions in the prefrontal cortex, striatum, hippocampus, and substantia nigra. A, sham-operation group; B, sham-operation and treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the TBI-induced group.
DISCUSSION
After TBI, cognitive impairments such as problems with memory, orientation, attention, executive functions, and problem-solving are often noticeable and long lasting (Griesbach et al., 2009). Treadmill exercise ameliorated impairments of short-term and spatial learning memories and improved depressive symptoms (Ko et al., 2018; Lee et al., 2018). Treadmill exercise was also effective for alleviation of TBI-induced memory loss (Kim et al., 2010). In the present study, the step-down avoidance task showed that TBI aggravated short-term memory. In addition, radial 8-arm maze test showed that TBI impaired spatial learning memory. In contrast, treadmill exercise alleviated the TBI-induced impairments of short-term and spatial learning memories in rats.
Dopamine is known to be a necessary substance for attention, behavioral outcome, executive function, and memory. Alterations in dopamine level have been suggested as the underlying mechanism of TBI pathology (Bales et al., 2009), but the exact mechanisms of TBI remain unclear. Dopamine plays an important role in regulating synaptic plasticity and storage information (Li et al., 2003). TH is a rate-limiting enzyme in the biosynthesis of dopamine, and TH is progressively decreased with the loss of dopaminergic neurons (Sung et al., 2012). Physical exercise is known to protect dopamine cell loss and increase dopamine levels in Parkinson’s disease and stress states (Mabandla et al., 2009). In the present study, the expressions of TH in the PFC, striatum, hippocampal dentate gyrus, and SN were decreased in the TBI rats, whereas treadmill exercise increased these TH expressions in the TBI rats. The present results show that an increase in dopamine level by treadmill exercise can contribute to the improvement of symptoms observed in the TBI rats.
D2 dopamine receptor is involved in memory consolidation and memory retention (Lénárd et al., 2017). Impaired D2 dopamine receptor function is also associated with decreased physical activity (Klinker et al., 2013). Decrease dopamine level was closely associated with increased D2 dopamine receptor expression in attention-deficit hyperactivity disorder rats (Ko et al., 2013). Swimming exercise improved attention deficit hyperactivity disorder symptoms by up-regulation of dopamine level and down-regulation of D2 dopamine receptor expression in attention-deficit hyperactivity disorder rats (Ko et al., 2013). Genetic variations of D2 might be associated with memory and attention tasks following mild TBI (McAllister et al., 2005). In the present study, the expressions of D2 dopamine receptor in the PFC, striatum, hippocampus, and SN were increased following TBI. Increased D2 dopamine receptor in the TBI rats might be considered as the compensatory mechanism for the reduced dopamine level caused by TBI. In contrast, treadmill exercise suppressed the D2 dopamine receptor expressions in the PFC, striatum, hippocampus, and SN of TBI rats. The suppressive effect of treadmill exercise on D2 dopamine receptor expression may be related to an improvement in memory functions of TBI rats.
In conclusion, TBI deteriorated short-term and spatial learning memories, in contrast, treadmill exercise alleviated the TBI-induced memory impairments by up-regulating dopamine level and down-regulating D2 dopamine receptor expression. Thus, treadmill exercise can be considered as a useful therapeutic strategy for relieving TBI symptoms.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant Funded by the Korean Government (NRF-2011-327-G00121).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Archer T, Svensson K, Alricsson M. Physical exercise ameliorates deficits induced by traumatic brain injury. Acta Neurol Scand. 2012;125:293–302. doi: 10.1111/j.1600-0404.2011.01638.x. [DOI] [PubMed] [Google Scholar]
- Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci Biobehav Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Crosson B, Halland K. Subcortical functions in cognition: toward a consensus. J Int Neuropsychol Soc. 2003;9:1027–1030. [Google Scholar]
- Granado N, Ortiz O, Suárez LM, Martín ED, Ceña V, Solís JM, Moratalla R. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cereb Cortex. 2008;18:1–12. doi: 10.1093/cercor/bhm026. [DOI] [PubMed] [Google Scholar]
- Griesbach GS. Exercise after traumatic brain injury: is it a double-edged sword? PM R. 2011;3(6 Suppl 1):S64–S72. doi: 10.1016/j.pmrj.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EY, Tsui PF, Kuo TT, Tsai JJ, Chou YC, Ma HI, Chiang YH, Chen YH. Amantadine ameliorates dopamine-releasing deficits and behavioral deficits in rats after fluid percussion injury. PLoS One. 2014;9:e86354. doi: 10.1371/journal.pone.0086354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Ko IG, Kim BK, Kim TW, Kim SE, Shin MS, Kim CJ, Kim H, Kim KM, Baek SS. Treadmill exercise inhibits traumatic brain injury-induced hippocampal apoptosis. Physiol Behav. 2010;101:660–665. doi: 10.1016/j.physbeh.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Klinker F, Hasan K, Paulus W, Nitsche MA, Liebetanz D. Pharmacological blockade and genetic absence of the dopamine D2 receptor specifically modulate voluntary locomotor activity in mice. Behav Brain Res. 2013;242:117–124. doi: 10.1016/j.bbr.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Ko IG, Kim SE, Hwang L, Jin JJ, Kim CJ, Kim BK, Kim H. Late starting treadmill exercise improves spatial leaning ability through suppressing CREP/BDNF/TrkB signaling pathway following traumatic brain injury in rats. J Exerc Rehabil. 2018;14:327–334. doi: 10.12965/jer.1836248.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko IG, Kim SE, Kim TW, Ji ES, Shin MS, Kim CJ, Hong MH, Bahn GH. Swimming exercise alleviates the symptoms of attention-deficit hyperactivity disorder in spontaneous hypertensive rats. Mol Med Rep. 2013;8:393–400. doi: 10.3892/mmr.2013.1531. [DOI] [PubMed] [Google Scholar]
- Lee JM, Ji ES, Kim TW, Kim CJ, Shin MS, Lim BV, Chung YR, Cho YS. Treadmill exercise improves memory function by inhibiting hippocampal apoptosis in pilocarpine-induced epileptic rats. J Exerc Rehabil. 2018;14:713–723. doi: 10.12965/jer.36394.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lénárd L, Ollmann T, László K, Kovács A, Gálosi R, Kállai V, Attila T, Kertes E, Zagoracz O, Karádi Z, Péczely L. Role of D2 dopamine receptors of the ventral pallidum in inhibitory avoidance learning. Behav Brain Res. 2017;321:99–105. doi: 10.1016/j.bbr.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Mabandla MV, Kellaway LA, Daniels WM, Russell VA. Effect of exercise on dopamine neuron survival in prenatally stressed rats. Metab Brain Dis. 2009;24:525–539. doi: 10.1007/s11011-009-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massucci JL, Kline AE, Ma X, Zafonte RD, Dixon CE. Time dependent alterations in dopamine tissue levels and metabolism after experimental traumatic brain injury in rats. Neurosci Lett. 2004;372:127–131. doi: 10.1016/j.neulet.2004.09.026. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Rhodes CH, Flashman LA, McDonald BC, Belloni D, Saykin AJ. Effect of the dopamine D2 receptor T allele on response latency after mild traumatic brain injury. Am J Psychiatry. 2005;162:1749–1751. doi: 10.1176/appi.ajp.162.9.1749. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144:1141–1151. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R, Loane DJ, Faden AI. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol Dis. 2013;54:252–263. doi: 10.1016/j.nbd.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockswold SB, Rockswold GL, Zaun DA, Zhang X, Cerra CE, Bergman TA, Liu J. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg. 2010;112:1080–1094. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- Sung YH, Kim SC, Hong HP, Park CY, Shin MS, Kim CJ, Seo JH, Kim DY, Kim DJ, Cho HJ. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson’s disease mice. Life Sci. 2012;91:1309–1316. doi: 10.1016/j.lfs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Scanlon JM, Becker CR, Ritter AC, Niyonkuru C, Dixon CE, Conley YP, Price JC. The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J Cereb Blood Flow Metab. 2014;34:1328–1339. doi: 10.1038/jcbfm.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jiang X. Effects of enteral nutrition on the barrier function of the intestinal mucosa and dopamine receptor expression in rats with traumatic brain injury. JPEN J Parenter Enteral Nutr. 2015;39:114–123. doi: 10.1177/0148607113501881. [DOI] [PubMed] [Google Scholar]