Abstract
The detrimental health effects of excessive alcohol consumption are well documented. Alcohol-induced liver disease (ALD) is the leading cause of death from chronic alcohol use. As with many diseases, the etiology of ALD is influenced by how the liver responds to other secondary insults. The molecular circadian clock is an intrinsic cellular timing system that helps organisms adapt and synchronize metabolism to changes in their environment. The clock also influences how tissues respond to toxic, environmental, and metabolic stressors, like alcohol. Consistent with the essential role for clocks in maintaining health, genetic and environmental disruption of the circadian clock contributes to disease. While a large amount of rich literature is available showing that alcohol disrupts circadian-driven behaviors and that circadian clock disruption increases alcohol drinking and preference, very little is known about the role circadian clocks play in alcohol-induced tissue injuries. In this review, recent studies examining the effect alcohol has on the circadian clock in peripheral tissues (liver and intestine) and the impact circadian clock disruption has on development of ALD are presented. This review also highlights some of the rhythmic metabolic processes in the liver that are disrupted by alcohol and potential mechanisms through which alcohol disrupts the liver clock. Improved understanding of the mechanistic links between the circadian clock and alcohol will hopefully lead to the development of new therapeutic approaches for treating ALD and other alcohol-related organ pathologies.
Keywords: alcohol, circadian clock, desynchrony, liver, steatosis
INTRODUCTION
Circadian rhythms are behavioral and biological processes exhibiting robust time-of-day oscillations that cycle on a period of ~24 h. These rhythms help organisms anticipate and prepare for environmental changes throughout the day. Circadian rhythms are highly influenced by external cues to the body (light-dark cycle) and behavioral factors (feeding-fasting cycle). However, internal levels of control are also present as cellular rhythms in metabolism tend to align with but are not solely dependent on external cues. Many of these rhythms are partially controlled by an intracellular molecular “clock.” The medical and scientific importance of the molecular circadian clock in biology was recognized by the award of the 2017 Nobel Prize in Physiology or Medicine to Drs. Jeffrey Hall, Michael Rosbash, and Michael Young for their pioneering work identifying the circadian cycling of the clock gene period in fruit flies (5, 83). Mounting evidence shows mechanistic links between the circadian clock and health and disease. The importance of the molecular clock in maintaining health is demonstrated by the fact that mice with genetically deleted or mutated clock genes throughout the entire body suffer numerous pathologies (68, 82). Similarly, humans with clock gene polymorphisms or environmentally disrupted circadian clocks from night-shift work are at higher risk for obesity and cardiovascular disease (35, 59). Recent findings linking circadian clock disruption in the liver to alcohol-induced liver disease (ALD) will be presented in this minireview article.
ALCOHOL AND LIVER DISEASE
Heavy and long-term alcohol use is connected to liver, lung, and heart disease, some cancers, myopathy, pancreatitis, immune dysfunction, and various cognitive impairments. ALD remains the number one cause of death from alcohol consumption in the US (33). Sadly, there are no Food and Drug Administration-approved drugs for treating ALD. These dire statistics emphasize the need for continued investigation into the molecular mechanisms of ALD so that more effective medical therapies can be developed for patients.
Alcohol can cause a number of liver diseases, including the early steatosis (fatty liver) stage and the more chronic and severe conditions of steatohepatitis (steatosis with inflammation and necrosis), fibrosis/cirrhosis, and hepatocellular carcinoma. Tissue injury is caused by oxidative and nitrative damage, redox imbalances, epigenetic modifications, inflammation, and fibrogenesis (52). Alcohol use also disrupts hepatic energy metabolism and impedes ATP synthesis by lowering glycogen content, decreasing glycolytic activity, and impairing mitochondrial bioenergetics (11). Dysregulation of key signaling and metabolic pathways contributes to steatosis via increased de novo lipogenesis, decreased β-oxidation of fatty acids, and impaired hepatocyte lipid export (79). These pathways and many others implicated in the etiology of ALD exhibit diurnal variations that can be influenced by the molecular circadian clock (47). For example, diurnal changes in the levels of the peroxisome proliferator-activated receptor (PPAR) family occur in the liver (75) along with rhythmicity of the transcriptional coactivators PPAR-γ coactivator-1α and -1β (PGC-1α and PGC-1β) (31, 58). It is believed that day-night differences in β-oxidation of fatty acids are driven by concerted cycle of PPARα and PGC-1α and clock-driven changes in mitochondrial protein acetylation (30, 42). Day-night differences in de novo lipogenesis are mediated in part through circadian clock-mediated control of sterol regulatory element binding protein-1c (SREBP-1c) and its various downstream targets (17), including genes involved in triglyceride synthesis and breakdown (1). Moreover, diurnal variations in lipid packaging and export from liver also appear to be regulated by the circadian clock through activation of the small heterodimer partner (SHP), which controls expression of the microsomal triglyceride transfer protein (MTP) (40). Importantly, many of these clock-controlled metabolic pathways are disrupted by alcohol consumption. Thus increased study on how alcohol perturbs circadian clock function will significantly improve our understanding of the mechanistic metabolic alterations that lead to ALD.
THE MOLECULAR CIRCADIAN CLOCK SYSTEM
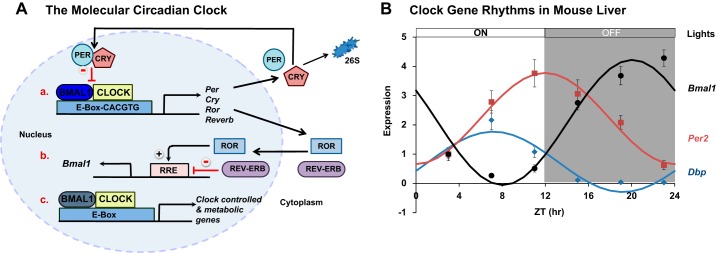
The molecular clock is a fundamental biological timing system comprised of several interconnected transcriptional-translational feedback loops (Fig. 1A). An excellent in-depth discussion on the mammalian circadian clock system is provided by Dr. Joseph Takahashi in Ref. 66. Briefly, the transcription factor brain and muscle aryl hydrocarbon nuclear translocator-like 1 (BMAL1) and a pair of Per-Arnt-Sim (PAS) domain helix-loop-helix proteins circadian locomotor output cycles kaput (CLOCK) or its paralog neuronal PAS domain protein 2 (NPAS2) comprise the core elements of the mammalian clock. On the positive side of this feedback loop, BMAL1 and CLOCK (or NPAS2) heterodimerize and bind to E-box DNA sequences (CACGTT) in the promoters of target genes (Fig. 1Aa). Two key targets of BMAL1 and CLOCK are the clock genes Period (Per1–3) and Cryptochrome (Cry1–2). DNA binding of BMAL1-CLOCK activates transcription of Per and Cry genes, which function as the negative arm of the clock feedback loop. PER and CRY dimerize and translocate back into the nucleus to inhibit DNA binding of BMAL1-CLOCK, thereby turning off their own transcription (Fig. 1Aa). Robust diurnal oscillations of the clock genes Bmal1 and Per2 and the clock-controlled gene D-site of albumin promoter binding protein (Dbp) in liver are shown in Fig. 1B. A second transcriptional-translational feedback loop involving opposing actions of the nuclear receptors retinoid acid receptor-related orphan receptors α, β, and γ (RORα, β, or γ) and nuclear receptor subfamily 1, group D, member 1 (NR1D1, also known as REV-ERBα) and 2 (NR1D2, also known as REV-ERBβ) is also important for maintaining normal clock activity as members of this loop jointly regulate Bmal1. In this loop, ROR activates, whereas REV-ERB inhibits Bmal1 transcription (Fig. 1Ab). BMAL1-CLOCK also activates rhythmic transcription of numerous noncore clock, transcription factor, and other metabolic genes (Fig. 1Ac). Up to 10% the liver transcriptome has been estimated to oscillate during the day with many of these oscillations controlled by the clock (41). In addition, temporal activity of the clock is regulated by other nontranscriptional and metabolic mechanisms (46). It is likely that alcohol-induced disruptions in any of these regulatory mechanisms could alter clock activity and clock-controlled pathways that are implicated in the initiation and progression of ALD. Some possible mechanisms are proposed later in this review.
Fig. 1.
The molecular circadian clock system and hepatic clock gene rhythms. A: the molecular circadian clock mechanism comprises transcriptional-translational feedback loops that control 24-h rhythms in clock genes and numerous other clock-controlled genes. At the core of the clock mechanism is the BMAL1-CLOCK heterodimer that activates transcription of multiple clock genes including components that comprise the negative feedback loops (PER, CRY, and REV-ERB), the positive feedback loop (ROR), and the transcription factors that regulate Bmal1 expression (ROR and REV-ERB). These feedback loops also regulate transcription of numerous non-clock metabolic genes. B: diurnal oscillations of Bmal1, Per2, and Dbp mRNA levels in livers from wild-type C57BL/6J male mice fed a normal chow diet. Livers were collected every 4 h [Zeitgeber time (ZT) 0 = lights on, ZT 12 = lights off]. Data significantly fit to a cosine function and represent the means ± SE for n = 4–6 mice per time point.
Circadian control of metabolism occurs by the interaction of clocks in different tissues. Communication between the principal or central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus and peripheral clocks located in all tissues of the body influences metabolism. The SCN clock is mainly reset by light (signal transmitted via the retinohypothalamic tract to SCN), whereas peripheral clocks are synchronized to the light-dark cycle and the SCN clock through various neural, peptide, and hormone connections (34). Peripheral tissue clocks can also be adjusted by other factors, including timing of food intake and metabolite fluxes (23), thus initiating dynamic shifts in rhythmic metabolic processes that are independent of the SCN. One critical function of clocks is to optimize the timing of energy metabolism (storage and utilization) to ensure proper cellular and whole body physiology. Again, this is accomplished by coordinated cycling of clock activators and repressors and their downstream metabolic and signaling targets (Fig. 1). This keeps multiple interconnected and complementary metabolic programs in sync within the cellular environment. Normal synchrony between the central clock and peripheral tissue clocks can be disrupted by environmental and behavioral disturbances. For example, restricting food access to the light (less active/sleep) period of the day in nocturnal rodents causes peripheral tissue clocks (e.g., liver clock) to shift their rhythms, while the SCN clock rhythm is largely unaffected (13). This induces a condition of circadian desynchrony between the central clock and peripheral tissues clocks. Circadian desynchrony has been associated with obesity in mice (22, 38). Moreover, simply feeding mice a high-fat diet (ad libitum) attenuates the amplitude of clock gene rhythms in liver and adipose tissue (22). Importantly, circadian desynchrony and disruptions in normal diurnal oscillations of clock components are now associated with various metabolic diseases.
The circadian clock is also proposed to regulate how tissues respond to toxicant exposure or secondary metabolic stress. Circadian clock disruption in the heart alters cardiac metabolism and promotes cardiomyopathy (80), whereas liver clock disruption reduces susceptibility to acetaminophen hepatotoxicity by downregulating CYP450 activity and formation of toxic acetaminophen metabolites (20). Given the growing recognition that clocks influence responsiveness of peripheral tissues to injurious agents and stress, several laboratories have begun to investigate the importance of clocks in alcohol-related tissue injury. Results from select experimental animal and human studies are presented in the remaining sections of this review along with a brief discussion about the implications these findings have for improved understanding of the role of the clock in ALD. A summary of these studies is provided in Table 1.
Table 1.
Alcohol-induced changes to the circadian clock and clock-regulated processes in experimental and human studies
| Model (Experimental or Clinical) | Alcohol-Feeding Model and Experimental Design | Outcomes | Reference |
|---|---|---|---|
| Clockdelta19 mouse | Alcohol in drinking water (15%) for 8 wk | Increased liver triglyceride; changed lipid and unchanged clock gene expression | 25 |
| Clockdelta19 mouse | Nanji liquid diet (29% ethanol calories) ± once weekly 12-h phase shifts in light-dark cycle for 8 wk | Increased gut leakiness and endotoxemia; altered day-night variations in intestinal tight junction proteins; increased hepatic steatosis | 61 |
| C57BL/6J and Per2Luc mice | Lieber-DeCarli liquid diet (28% ethanol calories) for 5 wk | Altered diurnal rhythms (phase, amplitude, mesor) in clock genes in liver, but not SCN; altered phase relationship between liver and SCN clocks (circadian desynchrony); altered metabolic gene rhythms in liver | 16 |
| C57BL/6J and Per2Luc mice | Lieber-DeCarli liquid diet (28% ethanol calories) for 1 and 4 wk | Altered diurnal rhythms in clock, lipid, and bile acid genes in liver; altered day-night differences in lipid metabolites and NAD+/NADH ratio | 85 |
| Per1Luc rat | Lieber-DeCarli liquid diet (35% ethanol calories) for 6 wk | Altered phase relationship between peripheral (adrenal and pituitary) clocks and SCN clock; phase advanced Per1 expression in adrenal and pituitary but not SCN | 18 |
| C57BL/6J mice | Lieber-DeCarli liquid diet (28% ethanol calories) for 5 wk | Altered diurnal rhythm in hepatic glycogen content; altered rhythms in glucose and glycogen metabolism genes and proteins (content and activity) | 69 |
| Hepatocyte-specific BMAL1 knockout mice (C57BL/6J background) | Lieber-DeCarli liquid diet (22% ethanol calories) for 5 wk | Arrhythmic hepatic glycogen content; altered diurnal rhythms in glucose and glycogen metabolism genes and proteins | 71 |
| Hepatocyte-specific BMAL1 knockout mice (C57BL/6J background) | Chronic + binge: Lieber-DeCarli liquid diet (35% ethanol calories) for 10 days + one “binge” (5 g/kg ethanol) on day 11 | Increased steatosis; altered de novo lipogenesis and fatty acid oxidation genes; rescue via administration of PPARα ligands | 84 |
| Shp−/−, Bhmt−/− mice and Shp−/−Bhmt−/− double knockout mice | Chronic + binge: Lieber-DeCarli liquid diet (35% ethanol calories) for 10 days + one “binge” (5 g/kg ethanol) on day 11 | Alcohol-induced HHCys is blunted in Shp−/− mice; increased steatosis in Bhmt−/−; partial rescue of HHCys by SHP deficiency in Shp−/−Bhmt−/− mice | 67 |
| Shp−/− mice and AAV8 REV-ERBα knockdown mice | Chronic + binge: Lieber-DeCarli liquid diet (35% ethanol calories) for 10 days + one “binge” (5 g/kg ethanol) on day 11 | Dysregulated lipid metabolism and steatosis in Shp−/− mice; genetic deletion of Shp and Rev-erba (double knockout) blocks alcohol-induced steatosis | 76 |
| Sprague-Dawley rats and Caco-2 cells | Rats: twice daily ethanol gavage (6 g/kg) for 2, 4, 8, and 10 wk Cells: 0.2% ethanol | Rats: increased gut leakiness and PER2 protein in duodenum and proximal colon Caco-2 cells: increased permeability of cell monolayer; increased CLOCK and PER2 protein; siRNA knockdown of CLOCK or PER2 prevents permeability | 62 |
| Clockdelta19 mouse | Nanji liquid diet (29% ethanol calories) for 10 wk | Altered gene expression (microarray study) in response to genotype and alcohol in liver, proximal colon, and hippocampus; largest number of genes affected in hippocampus | 60 |
| Human subjects | Patients with AUD and healthy controls | Decreased and fragmented sleep; lower plasma melatonin and increased intestinal permeability; increased blood LPS and LBP (endotoxemia) compared with healthy controls | 63 |
| Human subjects | DWs and NWs: 0.5 g/kg ethanol per day for 7 days | DLMO phased delayed in NWs; increased gut and whole gut permeability in NWs; altered clock gene expression in PBMCs in DWs and NWs with elevated inflammatory markers in NWs | 64 |
SCN, suprachiasmatic nucleus; PARP1, poly[ADP-ribose] polymerase-1; LBP, lipopolysaccharide-binding protein; HHCys, hyperhomocysteinemia; AUD, alcohol use disorder; DLMO, dim light melatonin onset; DWs, day-shift workers; NWs, night-shift workers; PBMCs, peripheral blood mononuclear cells.
LINKS BETWEEN CIRCADIAN CLOCK DISRUPTION AND ALD
Circadian clock disruption emerged early on as a contributor to alcohol use disorders and addiction. Several excellent reviews have been published describing the disruptive effects alcohol has on the central (SCN) circadian-timing system and circadian driven behaviors, as well as alcohol consumption and dependence (12, 45, 48); thus this work will not be discussed here. Of note, studies presented in these referenced reviews show that both circadian disruption by either genetic or environmental means increases alcohol preference and consumption in rodents. Importantly, some of these experimental findings do translate to humans. Long-term shift workers and individuals that work long shifts tend to engage in binge drinking (73). Genetic polymorphisms in some clock genes correlate with increased alcohol drinking and alcohol use disorders (24). Collectively, these studies and others support the hypothesis that circadian clock disruption may enhance participation in harmful alcohol-drinking behaviors, leading to disease.
Even with this rich literature on alcohol and clocks, only recently has the role of the circadian clock in alcohol-mediated tissue injury been explored. This work has however grown rapidly alongside research showing the importance of clocks in other metabolic diseases, like obesity-related liver disease. One of the earliest studies to examine the interaction of alcohol and clocks on liver injury was conducted by Kudo et al. (25) using the whole body Clock-mutant (Clockdelta19) mouse. They found increased triglyceride (steatosis) in livers of Clock-mutant mice provided alcohol in drinking water compared with wild-type mice. Alcohol increased acetyl CoA carboxylase 1 and decreased acyl CoA oxidase and MTP mRNA in livers of Clock-mutant mice. Changes in these lipid metabolism genes help to partially explain increased triglyceride content in livers of alcohol-fed Clock-mutant mice. However, these results are predicted as Clock-mutant mice are predisposed to obesity, dyslipidemia, steatosis, and many other behavioral and metabolic disorders (68). Moreover, as these mice are whole body mutants, there is no way of knowing whether increased steatosis in the alcohol-fed Clock-mutant mice was due to factors internal or external to the liver. In support of this last point, Summa et al. (61) demonstrated that Clock-mutant mice have increased intestinal gut leakiness and endotoxemia, factors known to promote ALD. Even with these limitations, these results are important as they support the hypothesis that clock disruption promotes ALD.
Following up on these studies, we examined the impact alcohol has on liver clock activity, as well as whether alcohol induces circadian desynchrony by altering the normal phase (timing) relationship between the SCN and liver clocks (16). For this, we used Per2::Luciferase (mPer2Luc) knockin mice fed nutritionally complete control or alcohol-containing liquid diets for a period of 5 wk to induce steatosis. In these mice, the Luc gene is fused in-frame to the 3′-end of the Per2 gene, thus serving as a real-time bioluminescent reporter of clock function in all tissues (77). Organ explants and tissue slices can be cultured using standard methods, and self-sustained PER2::LUC oscillations can be monitored for days to assess clock activity. Under normal control conditions, the SCN and liver clocks have a phase relationship of 3–4 h (77). This means that Per2 expression (as a readout of clock activity) peaks 3–4 h later in the liver than in the SCN. It is believed that this special timing relationship is critical for proper timing of activity/energy expenditure (controlled by SCN) to energy metabolism (controlled by liver). We found the alcohol feeding significantly decreases the phase between the SCN and the liver clock by advancing the peak of liver PER2::LUC rhythm, with no effect on timing of the PER2::LUC rhythm in the SCN (16). This result is important because it demonstrated for the first time that chronic alcohol feeding desynchronizes the liver clock from the SCN clock. We propose that this disruption contributes to the development of steatosis and other crucial metabolic disturbances in the liver following alcohol consumption.
We also measured clock gene expression rhythms in livers and SCN collected from wild-type C57BL/6J mice maintained on the same alcohol-feeding protocol for 5 wk (16). We measured mRNA levels of eight clock genes in livers collected at six different time points in one 24-h day. Cosinor analysis verified that all clock genes exhibited rhythmic expression in the liver. Importantly, alcohol-feeding phase advanced the peak of Rev-erba and Per2 in liver, thus replicating our findings seen in mPer2Luc mice. We also observed no effect of alcohol on clock gene rhythms in the SCN, again matching results seen in mPer2Luc mice. Alcohol feeding also decreased the mean expression (mesor) and amplitude of the rhythm for most of the clock genes. In a related study, Zhou et al. (85) also detected similar alcohol-mediated alterations to hepatic clock gene rhythms and circadian desynchrony between the liver and SCN clocks in alcohol-fed mPer2Luc mice. Interestingly, studies using the PER1::LUC rat also observed that alcohol-feeding phase advanced PER1::LUC rhythms in the adrenal and pituitary glands but not in the SCN (18). Collectively, these novel findings strongly support the hypothesis that alcohol desynchronizes peripheral tissue clocks from the central SCN clock.
Given these exciting findings, several areas require additional study. We still need to determine how alcohol alters circadian clock rhythms in the liver and how clock disruption in the liver contributes to alcohol-induced liver injury. With regards to the first question, it is highly probable that alcohol-mediated disruptions in hepatic metabolism feedback alter liver clock function (70). One likely candidate is the well-known alcohol-mediated change in the cellular redox state; i.e., the NAD+/NADH ratio, which is altered by alcohol metabolism. Precedent for a link between cellular redox state and the clock was first provided in studies showing that binding of the BMAL1-CLOCK/NPAS2 heterodimer to DNA is modulated by changes in pyridine nucleotide levels (49). High levels of NAD(P)H increase DNA binding of BMAL1-CLOCK, whereas NAD(P)+ decreases binding. Increases in NADH have also been shown to phase advance the clock (6), which fits nicely with our findings that the alcohol phase advances the liver clock (16). Notably, Zhou et al. (85) showed alcohol-mediated disruption in the phase and amplitude of the NAD+/NADH rhythm in livers of alcohol-fed mice. We have also observed an alcohol-induced shift in diurnal NAD+ and NADH rhythms (unpublished results). Alcohol-mediated alterations in NAD+ may also affect clock activity via modulating activities in the NAD+-dependent enzymes silent mating type information regulation 2 homolog 1 (SIRT1) and poly[ADP-ribose] polymerase 1 (PARP1). Studies show that these enzymes affect the amplitude and phase of clock rhythms through posttranslational modification (acetylation and PARylation) of clock proteins (2, 3). It is also possible that the depressed energy state in the alcohol-exposed liver (11, 81) disrupts the circadian clock system as clock proteins are also regulated by changes in ATP-dependent kinase-mediated phosphorylation. Multiple studies have shown that phosphorylation is important for setting the time and speed of the circadian clock (27, 72). For example, casein kinase 1 isoforms phosphorylate PER, CRY, and REV-ERB proteins affecting their subcellular localization, interaction, and/or stability (14, 15, 39). BMAL1 and CLOCK activity are also influenced by kinase-dependent phosphorylation (32, 50, 51). Moreover, AMP-activated protein kinase (AMPK) has been linked to regulation of the circadian clock by phosphorylation and degradation of CRY proteins (27). Importantly, alcohol-induced alterations in the activity of several of these key protein kinases, including AMPK and AKT, have been demonstrated in liver (53–55, 78). Accordingly, we propose that alcohol-mediated alterations in both the cellular redox and energy state likely contribute to clock dysregulation in the liver through a variety of mechanisms (Fig. 2).
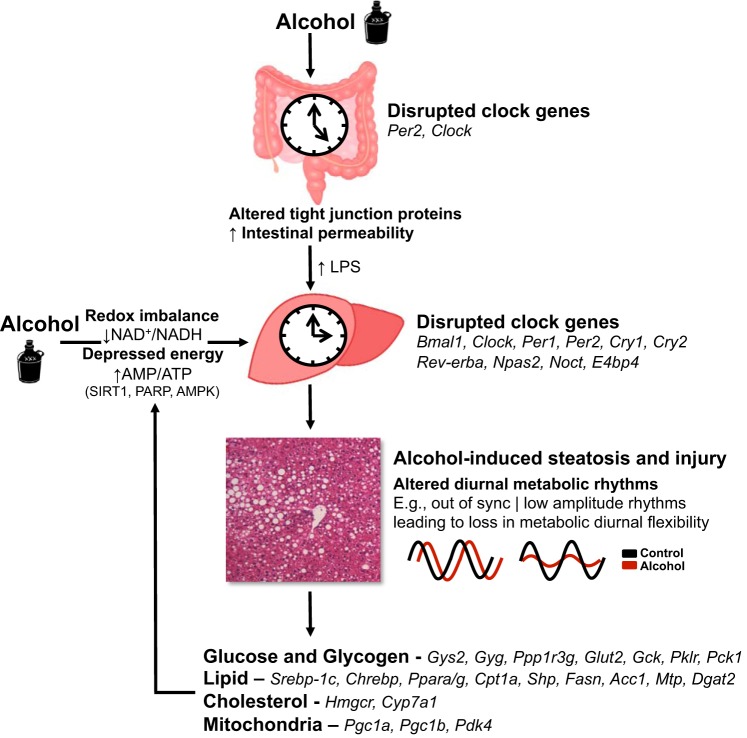
Fig. 2.
A hypothetical model for alcohol-mediated disruption of the liver circadian clock and liver injury. It is hypothesized that alcohol-mediated alterations in the circadian clock system contribute, in part, to the development of alcohol-induced steatosis and liver injury. There are many ways that alcohol might disrupt clock activity in the liver; herein, only a few hypothetical mechanisms are proposed. First, it is likely that alcohol-mediated alterations to intestinal clocks increases gut permeability, releasing LPS into the portal circulation with the ensuing alterations in hepatic inflammation and metabolism altering the liver clock. Second, alcohol-induced changes in the hepatic redox and energy state are also proposed to disrupt clock function. While the precise molecular mechanisms responsible for these alcohol-mediated effects on the clock are unknown, it is proposed that alterations in cellular redox may disrupt diurnal rhythms in BMAL1-CLOCK DNA binding along with the activities of the NAD+-dependent enzymes SIRT1 and PARP1, which modulate the phase and amplitude of circadian rhythms. Similarly, alcohol-mediated alterations in ATP-dependent kinase activities, e.g., AMPK, may negatively affect clock protein functions and timing. These alterations to the clock would, in turn, perturb clock-controlled rhythms in various downstream metabolic and/or signaling processes implicated in alcohol-induced liver injury, including, but not limited to, pathways in glycogen, lipid, cholesterol, and mitochondrial metabolism. Select targets are listed in the figure from cited papers. An alcohol-induced loss in flexible day-night rhythms in hepatic energy metabolism is predicted to cause a state of metabolic “inflexibility” in liver, thus hindering the liver’s ability to respond to changing energy demands throughout the day and perform critical cellular functions, including the repair of alcohol-induced tissue damage. It is also likely that alterations in these energy metabolism pathways might even “feedback” and further contribute to alcohol-mediated clock disruption thereby intensifying tissue injury.
In addressing the second question raised above, we and others have shown disrupted diurnal rhythms in multiple metabolic pathways in the alcohol-exposed liver. Alcohol feeding significantly alters diurnal rhythms in triglyceride, cholesterol, and bile acid metabolism, including fatty acid synthase (Fasn), cytochrome P450 7A1 (Cyp7a1), and 3-hydroxy-3-methylglutaryl-CoA-reductase (Hmgcr) (85). We observed that alcohol significantly dampens the diurnal rhythms of several key regulators of hepatic triglyceride, glycogen, and mitochondrial metabolism in mice that are clock regulated (16, 69, 71). For example, diurnal rhythms in Srebp-1c, Pgc-1a, Pparg, pyruvate dehydrogenase kinase 4 (Pdk4), phosphoenolpyruvate carboxykinase 1 (Pck1), and carnitine palmitoyltransferase 1a (Cpt1a) are completely lost in livers of alcohol vs. control-fed mice (16). We speculate that the dampening and/or loss in these essential energy metabolism rhythms cause a state of “metabolic inflexibility” in the liver, rendering the liver unable to meet changing energy and metabolic demands occurring throughout the day (Fig. 2). In support of this concept, we found that chronic alcohol and genetic disruption of the liver (hepatocyte) clock (i.e., BMAL1 deletion) abolishes the diurnal rhythm in hepatic glycogen, the cellular energy storage form of glucose, and the enzymes responsible for glycogen synthesis (71). Along these same lines, liver-specific deletion of BMAL1 enhances alcohol-mediated liver steatosis and injury by dysregulating fatty acid synthesis and oxidation (84). Wang and colleagues (40) have also identified novel roles of the SHP nuclear receptor in regulating daily rhythms in hepatic lipid and homocysteine metabolism following binge alcohol. The ability of SHP and the clock to influence triglyceride metabolism was first identified in studies showing SHP attenuates hypertriglyceridemia in Clock-mutant mice (40) and SHP interactions with NPAS2 to influence diurnal lipid metabolism rhythms in the liver (28). Moreover, genetic deletion of Shp and Rev-erba blocks alcohol-induced steatosis (76) and Shp knockout mice are protected from developing homocysteinemia following binge alcohol (67). While work in this area is still in the early stages, these results demonstrate that alcohol disturbs multiple metabolic pathways implicated in liver disease that are regulated by the circadian clock.
While this minireview has largely focused on the importance of clock disruption in promoting steatosis, circadian alterations likely contribute to other liver pathologies associated with chronic alcohol consumption. Genetic deletion of the clock gene Per2 in mice promotes cholestasis and fibrotic liver injury (9). Diurnal rhythms of clock gene transcripts are also altered in livers of carbon tetrachloride (CCl4)-treated mice (10). Similarly, Han et al. (19) reported elevated levels of clock genes in livers of bile-duct ligated (BDL) rats. Interestingly, constant darkness reduced biliary injury and fibrosis and normalized clock genes levels in livers from BDL rats presumably through a melatonin-based mechanism (19). Whether melatonin and/or phototherapy could provide some benefit against alcohol-induced biliary injury remains to be tested. Circadian clock gene disruption is also implicated in various liver cancers. Cholangiocarcinoma was significantly increased in livers of CRY1−/−/CRY2−/− double knockout compared with wild-type mice following diethylnitrosamine (DEN) treatment (36). The number of primary liver tumors was also increased in livers of PER2−/− mice compared with wild-type mice after DEN exposure (37). These findings suggest that clock genes might function as tumor suppressors. In related studies, environmental-induced circadian disruption from a chronic “jet-lag” model caused spontaneous hepatocellular carcinoma in wild-type mice through a mechanism involving dysregulation of bile acid signaling and promotion of fibrosis and cholestasis (21). Importantly, dysregulated levels of circadian clock gene transcripts have been reported in human hepatocellular carcinoma samples compared with normal livers (29). Finally, mice with genetic deletion of Rev-erbα develop more severe fulminant hepatitis compared with control mice following LPS/D-galactosamine injection due to enhanced inflammasome activity (44). These findings are of particular interest as inflammasome activation is implicated in alcohol-induced steatohepatitis (43). Taken together, these studies highlight the potential importance of circadian clock disruption and clock genes in all stages of ALD.
Strong associations also exist between alcohol-mediated damage to the gut and liver disease (65). Alcohol increases intestinal permeability, causing translocation of gut-derived endotoxin into the portal circulation. Endotoxin activates hepatic macrophages via Toll-receptor 4 signaling, leading to excess production of toxic cytokines and ultimately hepatocyte cell death. Building on this paradigm, Keshavarzian and colleagues (61) propose a role of intestinal cell clocks in ALD. As mentioned earlier, Clock-mutant mice have increased intestinal permeability, serum endotoxin levels, and increased liver injury following alcohol consumption (61). Companion studies found that environmentally-induced circadian clock disruption from weekly 12-h phase shifts in the light-dark cycle increase intestinal permeability and liver injury from alcohol in mice (61). These studies support the hypothesis of multiorgan circadian clock disruption in the etiology of ALD. With this said, it is interesting to note that while alcohol increases PER2 in the intestine (62), alcohol dampens clock gene rhythms in liver (16, 85). These results are intriguing because they suggest opposing effects of alcohol on the clock in different tissues. In fact, tissue-specific differences in expression patterns of various mRNAs are found in liver, colon, and hippocampus of alcohol-treated mice (60). Based on these results, it is recommended that multi-organ approaches should be used when studying alcohol’s impact on the clock, especially because alcohol disrupts the physiology and function of all organ systems in the body.
Notably, Keshavarzian and colleagues have begun to translate findings from their animal studies to humans. This is important as the majority of studies to date have examined the effects of alcohol on the circadian clock system using nocturnal rodent models (Table 1). Even though the main characteristics of circadian clock system are largely the same between nocturnal and diurnal animals as daily rhythms are controlled by the light-sensitive SCN clock (8), it is possible that alcohol-mediated effects on clocks and downstream metabolic targets may be dissimilar in nocturnal and diurnal animals (26). As such, it is critical that we begin to study whether results collected in experimental animals studies translate to humans. Swanson et al. (63) reported that individuals with alcohol use disorders exhibit circadian disruption as assessed by low plasma melatonin levels that are correlated with increased intestinal permeability. Moreover, in follow-up studies they found that while moderate alcohol drinking (0.5 g·kg−1·day−1 for 7 days) had no effect on sleep parameters in day- or night-shift workers, alcohol induces a 2-h phase delay in the dim light melatonin onset in night-shift workers, indicating alcohol-induced central circadian clock disruption (64). Accompanying this, alcohol increased colonic permeability in night, but not day-shift workers and altered the amplitude of clock gene rhythms in peripheral blood mononuclear cells from both day and night-shift workers. While preliminary, the results of these studies are exciting as they recapitulate earlier findings in animal studies (61) and importantly implicate night-shift work as a risk factor for alcohol-induced intestinal damage and possibly ALD.
SUMMARY AND PERSPECTIVES
The past 5 yr have provided increased interest and understanding of circadian clock disruption in alcohol-induced tissue injury. This research is based on the premise that alcohol-induced disruption in the circadian clock perturbs signaling and metabolism resulting in tissue pathology. As presented above, several independent research groups have demonstrated that alcohol disrupts the circadian clock in the liver and induces circadian desynchrony between the liver clock and the central SCN clock. Importantly, the impact of alcohol on peripheral tissue clocks is not only limited to the liver but also intestine, adrenal, and pituitary glands, thus highlighting the need to take a multiorgan approach in future studies. Indeed, testing whether alcohol desynchronizes the liver clock from other peripheral organ clocks (gut, adipose, muscle) and other brain region clocks (hippocampus), organs all implicated in regulating whole body energy metabolism, may reveal novel mechanisms of alcohol toxicity. It is also imperative that mechanisms responsible for alcohol-mediated clock disruption are identified. While alterations in cellular redox and energy state are proposed (Fig. 2), it is highly probable that clock disruption is mediated through other mechanisms, including oxidative stress, as well as posttranscriptional and other posttranslational alterations in the alcohol-exposed liver. Moving forward, studies should also take advantage of mouse models with cell-specific alterations in clock components to uncover the specific role of each cellular clock in alcohol-induced organ damage. Similarly, environmental models of circadian clock disruption should be included in more alcohol studies as these protocols have tremendous translational potential due to the growing number of studies showing associations between night-shift work and metabolic disease risk.
As the specific mechanisms responsible for alcohol-mediated clock disruption are identified, it may be possible to target the circadian clock system in the treatment of ALD. Chances for success are high as several pharmacological agents targeting the clock show some success in treating obesity-related diseases (57). Examples include several synthetic ligands targeting the nuclear receptors REV-ERB and ROR, which regulate Bmal1 expression. Studies show that these agents improve dyslipidemia and insulin sensitivity, augment mitochondrial biogenesis, and decrease inflammation in several different animal models of disease (7, 56, 57, 74). Even more intriguing is the possibility of developing drugs and other interventions, which not only prevent harmful alcohol-drinking behavior but also tissue injury. Development of potential “crossover” drugs targeting the clock that treat both alcohol-driven behaviors and pathology could be highly beneficial for patients. Finally, “time-of-day” should be investigated as an independent risk factor for ALD. Based on the vast literature on circadian regulation of metabolism, it is highly probable that drinking alcohol at different times of the day has distinct metabolic and pathological effects on liver and other organs. Similarly, considering “time” as a factor in alcohol studies might reveal times of day when drugs will be more effective. Timed or “chrono”-chemotherapy benefits cancer patients (4); thus there is every reason to believe that taking a chrono-therapy approach could also benefit ALD patients.
In conclusion, increased investigation into clocks and alcohol is highly beneficial as it will stimulate greater interdisciplinary collaborations between liver and brain/circadian researchers in the alcohol field, broaden and deepen our understanding of the pathobiology of ALD, and motivate scientists and clinicians to work together to develop and test new drugs and therapies for treating patients suffering with ALD.
GRANTS
S. M. Bailey’s circadian-focused research projects are supported by National Institute on Aging Grants R21-AA-020199 and R21-AA-024543. Additional research support is provided by the University of Alabama at Birmingham School of Medicine AMC21 Exploratory/Developmental Grant Application Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.B. prepared figures; S.M.B. drafted manuscript; S.M.B. edited and revised manuscript; S.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We kindly apologize to all investigators whose meritorious work in this area was not discussed or cited in this minireview because of space limitations.
REFERENCES
- 1.Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab 19: 319–330, 2014. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 3.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142: 943–953, 2010. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA. Systems chronotherapeutics. Pharmacol Rev 69: 161–199, 2017. doi: 10.1124/pr.116.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargiello TA, Jackson FR, Young MW. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312: 752–754, 1984. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- 6.Barnea M, Haviv L, Gutman R, Chapnik N, Madar Z, Froy O. Metformin affects the circadian clock and metabolic rhythms in a tissue-specific manner. Biochim Biophys Acta 1822: 1796–1806, 2012. doi: 10.1016/j.bbadis.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Billon C, Sitaula S, Burris TP. Inhibition of RORα/γ suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metab 5: 997–1005, 2016. doi: 10.1016/j.molmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 148: 5648–5655, 2007. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, Kakan X, Wang S, Dong W, Jia A, Cai C, Zhang J. Deletion of clock gene Per2 exacerbates cholestatic liver injury and fibrosis in mice. Exp Toxicol Pathol 65: 427–432, 2013. doi: 10.1016/j.etp.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Kakan X, Zhang J. Altered circadian rhythm of the clock genes in fibrotic livers induced by carbon tetrachloride. FEBS Lett 584: 1597–1601, 2010. doi: 10.1016/j.febslet.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham CC, Van Horn CG. Energy availability and alcohol-related liver pathology. Alcohol Res Health 27: 291–299, 2003. [PMC free article] [PubMed] [Google Scholar]
- 12.Damaggio AS, Gorman MR. The circadian timing system in ethanol consumption and dependence. Behav Neurosci 128: 371–386, 2014. doi: 10.1037/a0036408. [DOI] [PubMed] [Google Scholar]
- 13.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eide EJ, Vielhaber EL, Hinz WA, Virshup DM. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J Biol Chem 277: 17248–17254, 2002. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng GW, Edison, Virshup DM. Site-specific phosphorylation of casein kinase 1 δ (CK1δ) regulates its activity towards the circadian regulator PER2. PLoS One 12: e0177834, 2017. doi: 10.1371/journal.pone.0177834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filiano AN, Millender-Swain T, Johnson R Jr, Young ME, Gamble KL, Bailey SM. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PLoS One 8: e71684, 2013. doi: 10.1371/journal.pone.0071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilardi F, Migliavacca E, Naldi A, Baruchet M, Canella D, Le Martelot G, Guex N, Desvergne B; CycliX Consortium . Genome-wide analysis of SREBP1 activity around the clock reveals its combined dependency on nutrient and circadian signals. PLoS Genet 10: e1004155, 2014. doi: 10.1371/journal.pgen.1004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo R, Simasko SM, Jansen HT. Chronic alcohol consumption in rats leads to desynchrony in diurnal rhythms and molecular clocks. Alcohol Clin Exp Res 40: 291–300, 2016. doi: 10.1111/acer.12944. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Onori P, Meng F, DeMorrow S, Venter J, Francis H, Franchitto A, Ray D, Kennedy L, Greene J, Renzi A, Mancinelli R, Gaudio E, Glaser S, Alpini G. Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 307: G894–G904, 2014. doi: 10.1152/ajpgi.00288.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson BP, Walisser JA, Liu Y, Shen AL, McDearmon EL, Moran SM, McIntosh BE, Vollrath AL, Schook AC, Takahashi JS, Bradfield CA. Hepatocyte circadian clock controls acetaminophen bioactivation through NADPH-cytochrome P450 oxidoreductase. Proc Natl Acad Sci USA 111: 18757–18762, 2014. doi: 10.1073/pnas.1421708111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, Katchy CA, Lee C, Moore DD, Fu L. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30: 909–924, 2016. doi: 10.1016/j.ccell.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Kornmann B, Schaad O, Reinke H, Saini C, Schibler U. Regulation of circadian gene expression in liver by systemic signals and hepatocyte oscillators. Cold Spring Harb Symp Quant Biol 72: 319–330, 2007. doi: 10.1101/sqb.2007.72.041. [DOI] [PubMed] [Google Scholar]
- 24.Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lönnqvist J, Partonen T. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol 45: 303–311, 2010. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- 25.Kudo T, Tamagawa T, Shibata S. Effect of chronic ethanol exposure on the liver of Clock-mutant mice. J Circadian Rhythms 7: 4, 2009. doi: 10.1186/1740-3391-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar Jha P, Challet E, Kalsbeek A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol Cell Endocrinol 418: 74–88, 2015. doi: 10.1016/j.mce.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326: 437–440, 2009. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SM, Zhang Y, Tsuchiya H, Smalling R, Jetten AM, Wang L. Small heterodimer partner/neuronal PAS domain protein 2 axis regulates the oscillation of liver lipid metabolism. Hepatology 61: 497–505, 2015. doi: 10.1002/hep.27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Lu YF, Chen H, Liu J. Dysregulation of metallothionein and circadian genes in human hepatocellular carcinoma. Chronobiol Int 34: 192–202, 2017. doi: 10.1080/07420528.2016.1256300. [DOI] [PubMed] [Google Scholar]
- 30.Lin JD. Minireview: the PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol 23: 2–10, 2009. doi: 10.1210/me.2008-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 447: 477–481, 2007. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 32.Luciano AK, Zhou W, Santana JM, Kyriakides C, Velazquez H, Sessa WC. CLOCK phosphorylation by AKT regulates its nuclear accumulation and circadian gene expression in peripheral tissues. J Biol Chem 293: 9126–9136, 2018. doi: 10.1074/jbc.RA117.000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miniño AM, Heron MP, Smith BL. Deaths: preliminary data for 2004. Natl Vital Stat Rep 54: 1–49, 2006. [PubMed] [Google Scholar]
- 34.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35: 445–462, 2012. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer FAJL. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms 32: 154–164, 2017. doi: 10.1177/0748730417697537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mteyrek A, Filipski E, Guettier C, Oklejewicz M, van der Horst GT, Okyar A, Lévi F. Critical cholangiocarcinogenesis control by cryptochrome clock genes. Int J Cancer 140: 2473–2483, 2017. doi: 10.1002/ijc.30663. [DOI] [PubMed] [Google Scholar]
- 37.Mteyrek A, Filipski E, Guettier C, Okyar A, Lévi F. Clock gene Per2 as a controller of liver carcinogenesis. Oncotarget 7: 85832–85847, 2016. doi: 10.18632/oncotarget.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherji A, Kobiita A, Damara M, Misra N, Meziane H, Champy MF, Chambon P. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc Natl Acad Sci USA 112: E6691–E6698, 2015. doi: 10.1073/pnas.1519807112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohba Y, Tei H. Phosphorylation of N-terminal regions of REV-ERBs regulates their intracellular localization. Genes Cells 23: 285–293, 2018. doi: 10.1111/gtc.12571. [DOI] [PubMed] [Google Scholar]
- 40.Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab 12: 174–186, 2010. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. doi: 10.1016/S0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 42.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 122: 3476–3489, 2012. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pourcet B, Zecchin M, Ferri L, Beauchamp J, Sitaula S, Billon C, Delhaye S, Vanhoutte J, Mayeuf-Louchart A, Thorel Q, Haas JT, Eeckhoute J, Dombrowicz D, Duhem C, Boulinguiez A, Lancel S, Sebti Y, Burris TP, Staels B, Duez HM. Nuclear receptor subfamily 1 group D member 1 regulates circadian activity of NLRP3 inflammasome to reduce the severity of fulminant hepatitis in mice. Gastroenterology 154: 1449–1464.e1420, 2018. doi: 10.1053/j.gastro.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prosser RA, Glass JD. Assessing ethanol’s actions in the suprachiasmatic circadian clock using in vivo and in vitro approaches. Alcohol 49: 321–339, 2015. doi: 10.1016/j.alcohol.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy AB, Rey G. Metabolic and nontranscriptional circadian clocks: eukaryotes. Annu Rev Biochem 83: 165–189, 2014. doi: 10.1146/annurev-biochem-060713-035623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinke H, Asher G. Circadian clock control of liver metabolic functions. Gastroenterology 150: 574–580, 2016. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 48.Rosenwasser AM. Chronobiology of ethanol: animal models. Alcohol 49: 311–319, 2015. doi: 10.1016/j.alcohol.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293: 510–514, 2001. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 50.Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One 5: e8561, 2010. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanada K, Okano T, Fukada Y. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J Biol Chem 277: 267–271, 2002. doi: 10.1074/jbc.M107850200. [DOI] [PubMed] [Google Scholar]
- 52.Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol 26: 1089–1105, 2011. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- 53.Shearn CT, Smathers RL, Backos DS, Reigan P, Orlicky DJ, Petersen DR. Increased carbonylation of the lipid phosphatase PTEN contributes to Akt2 activation in a murine model of early alcohol-induced steatosis. Free Radic Biol Med 65: 680–692, 2013. doi: 10.1016/j.freeradbiomed.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shearn CT, Smathers RL, Jiang H, Orlicky DJ, Maclean KN, Petersen DR. Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. J Nutr Biochem 24: 1436–1445, 2013. doi: 10.1016/j.jnutbio.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sid B, Verrax J, Calderon PB. Role of AMPK activation in oxidative cell damage: Implications for alcohol-induced liver disease. Biochem Pharmacol 86: 200–209, 2013. doi: 10.1016/j.bcp.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Solt LA, Banerjee S, Campbell S, Kamenecka TM, Burris TP. ROR inverse agonist suppresses insulitis and prevents hyperglycemia in a mouse model of type 1 diabetes. Endocrinology 156: 869–881, 2015. doi: 10.1210/en.2014-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485: 62–68, 2012. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA 104: 5223–5228, 2007. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sookoian S, Gemma C, Gianotti TF, Burgueño A, Castaño G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr 87: 1606–1615, 2008. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 60.Summa KC, Jiang P, Fitzpatrick K, Voigt RM, Bowers SJ, Forsyth CB, Vitaterna MH, Keshavarzian A, Turek FW. Chronic alcohol exposure and the circadian clock mutation exert tissue-specific effects on gene expression in mouse hippocampus, liver, and proximal colon. Alcohol Clin Exp Res 39: 1917–1929, 2015. doi: 10.1111/acer.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS One 8: e67102, 2013. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swanson G, Forsyth CB, Tang Y, Shaikh M, Zhang L, Turek FW, Keshavarzian A. Role of intestinal circadian genes in alcohol-induced gut leakiness. Alcohol Clin Exp Res 35: 1305–1314, 2011. doi: 10.1111/j.1530-0277.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swanson GR, Gorenz A, Shaikh M, Desai V, Forsyth C, Fogg L, Burgess HJ, Keshavarzian A. Decreased melatonin secretion is associated with increased intestinal permeability and marker of endotoxemia in alcoholics. Am J Physiol Gastrointest Liver Physiol 308: G1004–G1011, 2015. doi: 10.1152/ajpgi.00002.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Van Den Berg J, Murphy T, Raeisi S, Fogg L, Vitaterna MH, Forsyth C, Turek F, Burgess HJ, Keshavarzian A. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol Gastrointest Liver Physiol 311: G192–G201, 2016. doi: 10.1152/ajpgi.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 148: 30–36, 2015. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179, 2017. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsuchiya H, da Costa KA, Lee S, Renga B, Jaeschke H, Yang Z, Orena SJ, Goedken MJ, Zhang Y, Kong B, Lebofsky M, Rudraiah S, Smalling R, Guo G, Fiorucci S, Zeisel SH, Wang L. Interactions between nuclear receptor shp and foxa1 maintain oscillatory homocysteine homeostasis in mice. Gastroenterology 148: 1012–1023.e1014, 2015. doi: 10.1053/j.gastro.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Udoh US, Swain TM, Filiano AN, Gamble KL, Young ME, Bailey SM. Chronic ethanol consumption disrupts diurnal rhythms of hepatic glycogen metabolism in mice. Am J Physiol Gastrointest Liver Physiol 308: G964–G974, 2015. doi: 10.1152/ajpgi.00081.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Udoh US, Valcin JA, Gamble KL, Bailey SM. The molecular circadian clock and alcohol-induced liver injury. Biomolecules 5: 2504–2537, 2015. doi: 10.3390/biom5042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Udoh US, Valcin JA, Swain TM, Filiano AN, Gamble KL, Young ME, Bailey SM. Genetic deletion of the circadian clock transcription factor BMAL1 and chronic alcohol consumption differentially alter hepatic glycogen in mice. Am J Physiol Gastrointest Liver Physiol 314: G431–G447, 2018. doi: 10.1152/ajpgi.00281.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Virshup DM, Eide EJ, Forger DB, Gallego M, Harnish EV. Reversible protein phosphorylation regulates circadian rhythms. Cold Spring Harb Symp Quant Biol 72: 413–420, 2007. doi: 10.1101/sqb.2007.72.048. [DOI] [PubMed] [Google Scholar]
- 73.Virtanen M, Jokela M, Nyberg ST, Madsen IE, Lallukka T, Ahola K, Alfredsson L, Batty GD, Bjorner JB, Borritz M, Burr H, Casini A, Clays E, De Bacquer D, Dragano N, Erbel R, Ferrie JE, Fransson EI, Hamer M, Heikkila K, Jockel KH, Kittel F, Knutsson A, Koskenvuo M, Ladwig KH, Lunau T, Nielsen ML, Nordin M, Oksanen T, Pejtersen JH, Pentti J, Rugulies R, Salo P, Schupp J, Siegrist J, Singh-Manoux A, Steptoe A, Suominen SB, Theorell T, Vahtera J, Wagner GG, Westerholm PJ, Westerlund H, Kivimaki M. Long working hours and alcohol use: systematic review and meta-analysis of published studies and unpublished individual participant data. BMJ 350: g7772, 2015. doi: 10.1136/bmj.g7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Nevière R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19: 1039–1046, 2013. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810, 2006. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 76.Yang Z, Tsuchiya H, Zhang Y, Lee S, Liu C, Huang Y, Vargas GM, Wang L. REV-ERBα activates C/EBP homologous protein to control small heterodimer partner-mediated oscillation of alcoholic fatty liver. Am J Pathol 186: 2909–2920, 2016. doi: 10.1016/j.ajpath.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol 287: G1–G6, 2004. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 79.You M, Jogasuria A, Taylor C, Wu J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg Nutr 4: 88–100, 2015. doi: 10.3978/j.issn.2304-3881.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, Chatham JC, Wende AR, Yang Q, Chow CW, Martino TA, Gamble KL. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms 29: 257–276, 2014. doi: 10.1177/0748730414543141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young TA, Bailey SM, Van Horn CG, Cunningham CC. Chronic ethanol consumption decreases mitochondrial and glycolytic production of ATP in liver. Alcohol Alcohol 41: 254–260, 2006. doi: 10.1093/alcalc/agl017. [DOI] [PubMed] [Google Scholar]
- 82.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 3: 479–493, 2011. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zehring WA, Wheeler DA, Reddy P, Konopka RJ, Kyriacou CP, Rosbash M, Hall JC. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell 39: 369–376, 1984. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]
- 84.Zhang D, Tong X, Nelson BB, Jin E, Sit J, Charney N, Yang M, Omary MB, Yin L. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARα pathway. Hepatology 2018 Mar 13 [Epub ahead of print]. doi: 10.1002/hep.29878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou P, Ross RA, Pywell CM, Liangpunsakul S, Duffield GE. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci Rep 4: 3725, 2014. doi: 10.1038/srep03725. [DOI] [PMC free article] [PubMed] [Google Scholar]