Abstract
Substance use disorder is a leading causes of preventable disease and mortality. Drugs of abuse cause molecular and cellular changes in specific brain regions and these neuroplastic changes are thought to play a role in the transition to uncontrolled drug use. Neuroimaging has identified neural substrates associated with problematic substance use and may offer clues to reduce its burden on the patient and society. Here, we provide a narrative review of neuroimaging studies that have examined the structures and circuits associated with reward, cues and craving, learning, and cognitive control in substance use disorders. Most studies use advanced MRI or positron emission tomography (PET). Many studies have focused on the dopamine neurons of the ventral tegmental area, and the regions where these neurons terminate, such as the striatum and prefrontal cortex. Decreases in dopamine receptors and transmission have been found in chronic users of drugs, alcohol, and nicotine. Recent studies also show evidence of differences in structure and function in substance users relative to controls in brain regions involved in salience evaluation, such as the insula and anterior cingulate cortex. Balancing between reward-related bottom-up and cognitive-control-related top-down processes is discussed in the context of neuromodulation as a potential treatment. Finally, some of the challenges for understanding substance use disorder using neuroimaging methods are discussed.
Introduction
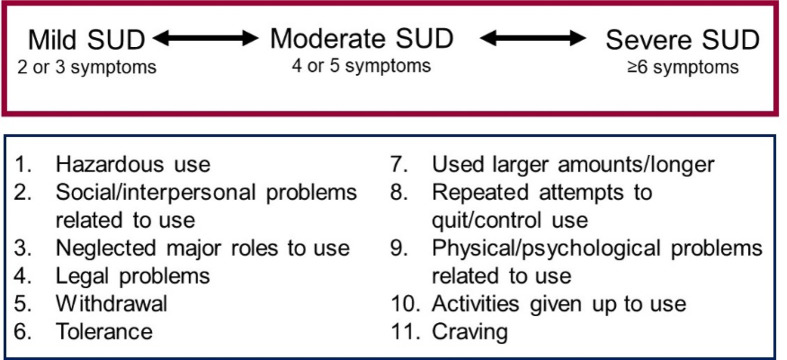
The prevalence of illicit drug use worldwide was 5.6% in 2016 and the harmful use of alcohol accounted for 5% of the global disease burden.1 In the United States, by the twelfth grade 49 percent of youth have tried an illicit drug and 62 percent have tried alcohol,2 but only a subgroup of individuals progress from use to an addiction, known clinically as a substance use disorder. A substance use disorder is defined as physical dependence, a diminished capacity to control one’s use despite negative consequences, and a strong craving to use the substance. A diagnosis of a substance use disorder (SUD) in the Diagnostic Statistical Manual-5th edition (DSM-5) is determined by the number of symptoms, with two or three symptoms representing mild and greater than six symptoms representing severe SUD3 (Figure 1).
Figure 1.
Substance use disorder (SUD) is a continuum based on 11 symptoms (Diagnostic Statistical Manual of Mental Disorders, fifth Edition, American Psychiatric Association).3
Risks for substance use and the development of SUD include person-level factors (e.g. genetic predisposition, socio-demographics, problems of inhibitory control, and other diagnoses, such as conduct disorder), family-level factors (e.g. older sibling use of substances, family history of addiction), school/community-level factors (e.g. neighborhood poverty and disorganization) and societal-level factors (e.g. laws, norms).3–11 Substance use disorders affect 1 in 10 adults annually,12,13 exert a heavy toll on patients, their families, and society,14 and are common causes of preventable death.15,16 Although once considered the result of a “moral failing,” in recent decades the neuroscience of addiction has clearly demonstrated a biological basis of the illness.17
Because imaging has played a significant role in this shift in perception, it is important that radiologists understand the evidence, pathophysiology, and treatment implications. Understanding the neuroanatomy of substance related behavior is necessary for future potential image-guided therapies. Patients and other physicians frequently look to imaging for answers about SUD and radiologists should be able to give informed guidance. Finally, radiologists should understand how imaging provides insights into mechanisms of behavior. With the clinical neuroradiologist in mind, this review seeks to answer “How do brain structure and function contribute to substance use disorder?” and “What does knowledge of the brain’s role in substance use disorder imply for future treatment?”
preclinical studies of drug reward and neuroplasticity
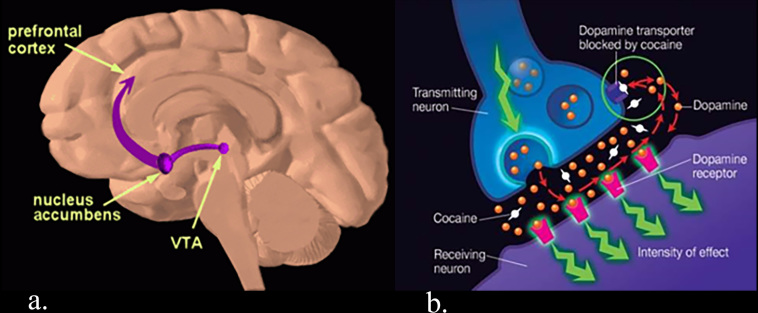
A central tenet in substance use research is that exposure to drugs is associated with neuroplasticity. These neuroplastic changes are thought to underpin behaviors that, in some people, become pathological incentives to seek and procure drugs. All drugs of abuse cause the release of dopamine into the nucleus accumbens (Nac),18,19 producing a pleasurable feeling that reinforces use. The mesolimbic reward system consists of dopaminergic neurons with cell bodies in the ventral tegmental area of the midbrain and terminal projections in the ventral striatum (nucleus accumbens), prefrontal cortex, and limbic regions. Dopamine binds to post synaptic receptors and is then returned to the presynaptic axon by the dopamine transporter (DAT), terminating the signal. Cocaine and other drugs of abuse enhance dopamine signaling through several mechanisms, such as by inhibiting the dopamine transporter (Figure 2).
Figure 2.
(a) The mesolimbic reward pathway connects the ventral tegmental area (VTA) in the midbrain to the nucleus accumbens and prefrontal cortex. (b) Cocaine blocks the dopamine transporter (DAT) thereby preventing synaptic dopamine from being returned to the pre synaptic axon. (From the National Institute of Drug Abuse (NIDA); https://www.drugabuse.gov/publications/teaching‐packets/understanding‐drug‐abuse‐addiction/sectioni/4‐reward‐pathway and https://www.drugabuse.gov/publications/research‐reports/cocaine/how‐doescocaine‐ produce‐its‐effects).
Over time, cues - people, places and things - become associated with the initial hedonic effects of drug induced dopamine transmission and those cues can trigger craving, anticipatory euphoria, or even symptoms of withdrawal.20 Animal studies have shown that a single drug exposure can have a lasting effect. Mice that were given a single dose of cocaine showed greater sensitivity of excitatory synapses onto dopaminergic cells in the ventral tegmental area, and this sensitivity persisted for five days.21 Such substance-induced plasticity likely plays a role in the development of addiction, where normal reward processing is diverted towards drug-related rewards,22 leading to over valuing of drug. In rats trained to self-administer cocaine by pressing a lever, the behavior was extinguished after they stopped receiving cocaine in response to the lever-presses, but a single injection of cocaine led the rats to press the lever again, a phenomenon known as sensitization.23 A widely used behavioral paradigm in substance use research is condition place preference, a form of conditioning where animals learn to associate attributes of the cage with receipt of rewarding stimuli.
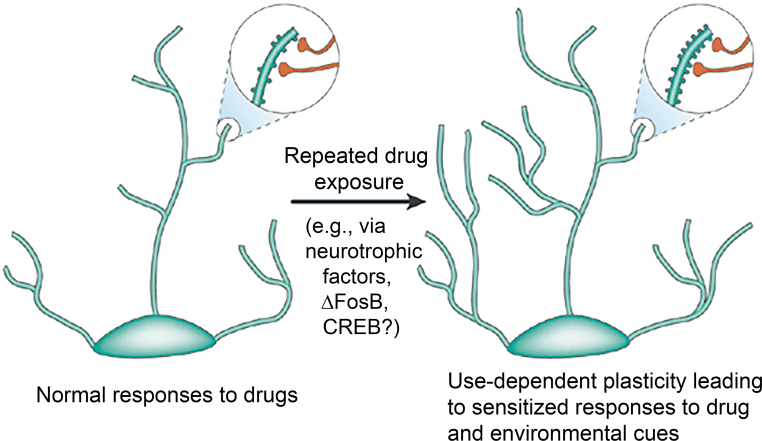
Sensitization and conditioning involve changes in cell signaling, gene expression, receptor density, and synaptic morphometry. Cocaine and opiates, for example, have been shown to change the morphology of dendritic spine density and number (Figure 3).24,25 Repeated exposure to drugs decrease frontal and striatal activity and cause a shift from ventral to dorsal striatal processing.26,27 These cellular and circuit changes, in aggregate, should be detectable with imaging allowing for the possibility that imaging may be a tool for diagnosis and management.
Figure 3.
Repeated drug exposure can alter neuronal structure through several mechanisms at the cellular level. (Reprinted from Nestler (2001) with permission from Springer Nature)24
PET and structural MRI in substance use disorder (SUD)
This paper is organized primarily around the behavioral phases of SUD, which will be covered in detail in section four and Table 1. Here, we review PET imaging techniques in substance use research because radiologists may be unfamiliar with these methods. Next, we provide a brief overview of findings from structural MRI studies due to the large body of evidence available. One caveat is that different drug classes are treated as a single entity, as a drug-specific review is beyond the scope of the paper. Another caveat is that an overview of DTI in SUD will not be presented.
Table 1.
Summary of neuroimaging papers on drug reward, cues and craving, learning, and cognitive control
| Ref | Date | Author | Journal | Acute Drug Admin | Subjects | n | Modality | Primary result |
| Drug reward | ||||||||
| 28 | 1997 | Volkow, et al. | Nature | Cocaine IV | CUD | 17 | PET [11C] cocaine | Blockade of DAT in striatum correlates with subjective high |
| 29 | 1998 | Stein, et al. | Am J Psych | Nicotine IV | Smoker | 16 | fMRI 1.5T | Dose-dependent ↑ BOLD in NAc, ACC, frontal lobes, amygdala and self-reported high |
| 30 | 1997 | Breiter, et al | Neuron | Cocaine IV | CUD | 10 | fMRI 1.5T | Early, short ↑ BOLD in VTA, caudate, ACC, pons, lateral frontal, basal forebrain correlates with rush |
| Non-drug reward | ||||||||
| 31 | 2017 | Luijten, et al | JAMA Psych | Multiple substances, alcohol, nicotine | 25 studies, 500 patients, 475 HC fMRI | Seed-based Meta | ↑ vs during reward outcome: ↓ vs during reward anticipation in patients vs HC | |
| 32 | 2010 | Asensio, et al | Synapse | CUD | seven active CUD | fMRI 4T monetary incentive task; PET [11C] Raclopride | ↓ D2 binding DS correlates with d BOLD in during monetary reward; ↓ D2 vs correlates with ↑ mPFC during monetary reward | |
| Cues and craving | ||||||||
| 33 | 2011 | Kuhn, et al | Eur J Neurosci | Smoker, AUD, CUD | 13 Nic studies, 251 subjects; 10 Alc studies, 112 subjects; six coc studies, 83 subjects | Meta drug cues | Nicotine, alcohol and cocaine cues: ↑ vs , ACC, amygdala. Craving correlated with ACC and vs . | |
| 34 | 2012 | Engelmann, et al | Neuroimage | Smokers | 11 studies, 200 subjects | Meta smoking cues | ↑visual, precuneus, posterior cingulate, dorsal and medial PFC, insula | |
| 35 | 2000 | Garavan, et al | Am J Psych | CUD | 17 CUD, 14 HC | 1.5T fMRI video | ↑ACC, right parietal, caudate | |
| 36 | 2016 | Kober, et al | Neuropsychopharm | CUD, gamblers | 30 CUD, 28 PG, 45 HC | 3T fMRI video | ↑ACC greater to cocaine video in CUD; ↑medial dorsal to gambling video gamblers vs CUD | |
| 37 | 2001 | Wexler, et al | Am J Psych | CUD | 11 CUD, 21 HC | 1.5T fMRI video | ↑ACC precedes cocaine craving; however, depends on level and timing relative to craving. | |
| 38 | 2011 | Wilcox, et al | Drug Alc Dep | CUD | 14 CUD, 16 HC | cocaine videos | ↑ left DLPFC and occipital | |
| 39 | 1999 | Childress, et al | Am J Psych | CUD | 14 CUD, 6 HC | [15O]-H2O PET | ↑amygdala, ACC | |
| Learning | ||||||||
| 40 | 2014 | Rose, et al | Neuropsychopharm | CUD | 14 CUD, 14 HC | 3T fMRI food reinforcement learning | ↑ signal to unexpected negative outcomes; ↓ signal to positive events in dopaminergic reward regions | |
| 41 | 2013 | Tanabe, et al | Am J Psych | SUD | 32 SUD, 30 HC | 3T fMRI monetary reinforcement learning | ↓vMPFC prediction error learning signal | |
| 42 | 2018 | Wang, et al | Biol Psych Cog Neurosci Imag | CUD | 22 CUD, 54 HC | 3T fMRI probabilistic loss-learning | ↑ vs positive PE learning signal in cocaine-deprived vs sated patients | |
| Cognitive control | ||||||||
| 43 | 2011 | Nestor, et al. | NeuroImage | Smoker | 10 ex-smoker, 13 smoker, 13 HC | 3T fMRI Cues and Go No-go | Cue reactivity: Smoker >HC ↑Nacc and amygdala; Motor inhibition: HC >ex-smoker>smoker ↑ ACC and left MFG and IFG | |
| 44 | 2013 | Camchong, et al. | Alc Clin Exp Res | AUD | 27 short term abstain (STAA), 23 long term abstain (LTAA), 23 HC | 3T fMRI resting state synchrony | ↑ RSS of executive control and ↓ RSS of reward processing in HC >STAA > LTAA: | |
| 45 | 2016 | Regner, et al. | PloS One | SUD | 50 abstinent SUD, 50 HC | 3T fMRI resting state effective connectivity | ↑ connectivity ECN→DMN→BG network in abstinent SUD | |
| 46 | 2015 | Hanlon, et al. | Brain Res | CUD | 11 CUD | cTBS mPFC | decreases activity in the striatum and anterior insula | |
ACC, anterior cingulate cortex; AUD, Alcohol use disorder; BG, basal ganglia network; BOLD, Blood oxygen-level-dependent; CUD, cocaine use disorder; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; ECN, executive control network; HC, healthy control; IFG = inferior frontal gyrus,MFG, middle frontal gyrus; NAc, nucleus accumbens; PE, prediction error; RSS, resting state synchrony; SUD, substance use disorder; VTA, ventral tegmental area; cTBS, continuous θ burst transcranial magnetic stimulation; vMPFC, ventral medial prefrontal cortex; vs, ventral striatum.
PET
Positron emission tomography (PET) can be used to image specific receptors and neurotransmitters and has played a critical role in our understanding of substance use disorders. The main outcome measure in PET studies is receptor availability or “binding potential” (BP), which is the ratio of specific binding to non-specific binding (specific binding refers to the radiotracer bound to the receptor of interest and non-specific binding refers to background binding).47
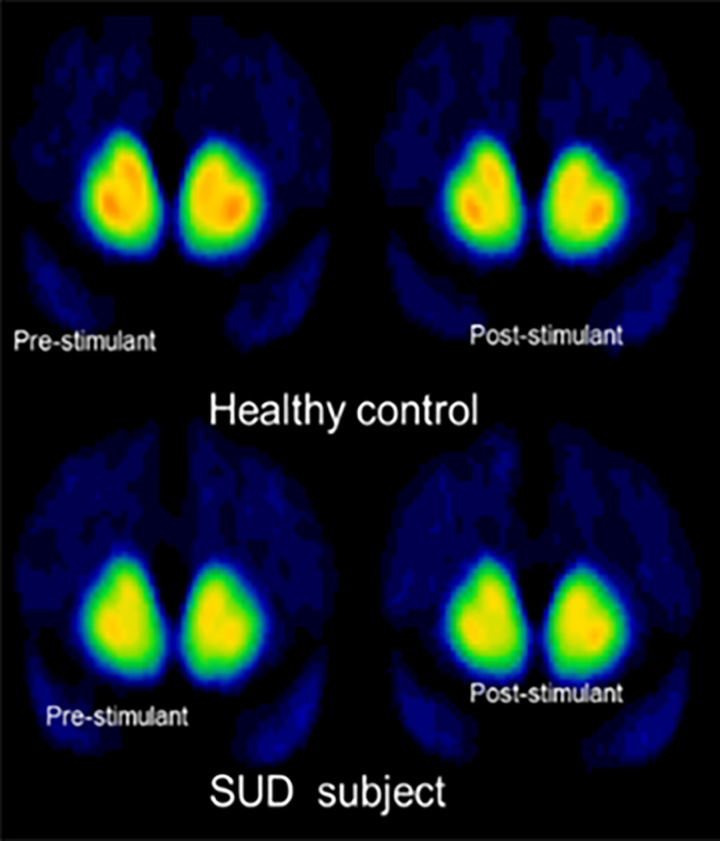
In substance use research, PET radiotracers have been developed to image dopamine Type 1 (D1), dopamine Type 2 (D2), opioid receptors (μ, κ, and δ), cannabinoid receptors (CB1 and CB2), and monoamine transporters. Of these, dopamine imaging has been the most extensively studied, particularly with respect to reward driven behavior. [11C]-raclopride is a radiotracer that measures D2 receptor BP but also allows imaging of presynaptic dopamine release. By imaging before and after the administration of a psychostimulant, such as amphetamine or methylphenidate, [11C]-raclopride can be used to visualize the amount of dopamine released in the brain in response to the stimulant. Stimulants cause dopamine levels to increase in the brain resulting in a decrease in D2 receptors available to bind to the radiotracer. As a result, a greater decrease in radiotracer binding correlates with more dopamine release. Thus, a number of PET imaging studies in drug and alcohol addiction use two outcome measures: (1) BP to measure D2 receptor availability and (2) the percent decrease in radiotracer binding (ΔBPND) which provides an estimate of endogenous dopamine release48 (Figure 4).
Figure 4.
PET scans in a healthy control (top row) and substance use disorder (SUD) subject (bottom row) scanned with [11C]raclopride at baseline (left) and after administration of a stimulant (right). Prestimulant baseline images demonstrate less D2 receptor binding (BP) in the SUD subject compared to the control. Post-stimulant images demonstrate less [11C] raclopride displacement (ΔBP) in the SUD subject compared to the control. Thus, D2 receptor binding (BP) and dopamine release (Δ BP) are blunted in substance use.
Structural MRI
Most readers will be familiar with voxel-based morphometry (VBM). While volume loss is well established for alcohol use disorder,49,50 studies using VBM methods have also demonstrated lower gray matter volumes in individual who abuse cocaine,51 amphetamine,52–54 and nicotine55,56 compared to healthy adults. Lower tissue volumes are most commonly reported in the anterior cingulate, medial frontal, dorsolateral prefrontal, and parietal cortices. Although a few studies have found increased subcortical gray matter volumes in stimulant dependence,57,58 the overall evidence supports lower cortical gray matter volumes. A meta-analysis of VBM studies in stimulant dependence showed grey matter volume deficits in the insula, thalamus, anterior cingulate, and ventral prefrontal cortex, but did not identify any regions with increased grey matter volumes.59 Differences in subcortical versus cortical changes associated with drugs remains a possible explanation, however. As the majority of these studies are cross-sectional, it remains unclear if differences in grey matter volume predispose individuals to, or result from, chronic drug exposure, but overall suggests that tissue volume is a biomarker of SUD.
imaging reward, cues and craving, learning, and cognitive control in SUD
A summary of the neuroimaging studies discussed in this section is found in Table 1.
Reward
While animal studies can measure the reinforcing properties of drugs, the pleasurable “high” can only be measured in humans. One of the first studies to investigate the subjective rewarding effects of cocaine used the radiotracer [11C]-cocaine which binds to the dopamine transporter (DAT). Compared to placebo, intravenous cocaine at doses used by humans caused a decrease [11C]-cocaine binding in the striatum compatible with cocaine blockade of the DAT. Increasing the dose from 0.3 to 0.6 mg/kg cocaine further reduced the radiotracer binding and correlated with greater self-reported high indicating a dose-response.28 Using fMRI, researchers found that acute administration of intravenous nicotine29 and cocaine30 both increased blood-oxygen-level-dependent (BOLD) signal in the striatum, amygdala and prefrontal cortex. Higher plasma nicotine levels corresponded to a larger signal increase in these regions.29 There were also regionally distinct temporal profiles of the BOLD signal. Short-duration increases in the ventral tegmental area, caudate and cingulate gyrus correlated with feelings of “rush”, while sustained signal increases in the NAc correlated with greater drug craving.30 Thus, PET and fMRI studies confirm that the mesolimbic system is involved in drug reward in humans.
What is the effect of chronic drug exposure on dopaminergic mesolimbic activity? PET imaging studies consistently report a decrease in striatal D2 receptor binding in SUD. This finding has been has been seen across many drugs, including cocaine, methamphetamine, opiate/opioid, nicotine, and alcohol use disorder. Many of these studies have also shown that addiction to drugs, alcohol, and nicotine are associated with lower dopamine release (ΔBP) compared to control subjects60–62 (Figure 4).
While the ventral striatum is clearly involved in the rewarding effects of acute drug, in chronic users the striatal response is altered in response to non-drug (i.e. hypothetical or real monetary) reward. A meta-analysis of 500 patients compared to 475 controls across multiple addictions found lower ventral striatal activity during the anticipation of money reward suggesting a blunted response to non-drug reward.31 In cocaine use disorder, the decrease in D2 receptor availability in the dorsal striatum has been shown to correlate with decreased thalamic response to a monetary reward (measured with fMRI) while low D2 binding in the ventral striatum was associated with increased medial prefrontal response to monetary reward.32 Thus, chronic drug exposure may interfere with processing of normal rewards through striatal dysfunction. Specifically, D2 receptor availability may predict regional differences in brain responses to a non-drug reward, consistent with anatomically and functionally specific regions in the striatum, a finding that may have treatment implications.
Cues and craving
Cues are a powerful trigger for drug and alcohol relapse. Brain regions activated by drug cues extend well beyond the mesolimbic reward system. In smokers, for example, cigarette cues were associated with activity in prefrontal cortex, insula, cingulate cortex, parietal, occipital, and temporal lobes, limbic system and basal ganglia.34 A meta-analysis found that smoking cues activated the extended visual system, which includes the lingual gyrus, fusiform gyrus and cuneus, possibly reflecting the allocation of resources toward visual association areas.34,63 The anterior cingulate and dorsomedial prefrontal cortices appear to be common foci of cue reactivity across many drugs, possibly because medial frontal-striatal regions assign saliency to stimuli. In cocaine use disorder (CUD), the anterior cingulate, medial prefrontal cortex, ventral striatum, and parietal lobe show greater activation in response to drug cues than to neutral cues.35–37 Compared to controls, subjects with CUD also showed greater reactivity to cocaine cues in the dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex.38 A meta-analysis of nicotine, alcohol, and cocaine studies found convergence in the ventral striatum, anterior cingulate cortex, and amygdala as regions that are more responsive to drug compared to neutral cues across these drugs.33 Collectively, these studies indicate that substance use involves altered neural activity in reward-related and cognitive control-related pathways in response to drug cues and non-drug reward, consistent with homeostatic shifts in reward processing.
Reinforcement learning
Maladaptive learning is a hallmark of SUD. Reinforcement (i.e. trial-and-error) learning requires one to predict outcomes, compare expected to actual outcomes, and update future predictions. Learning occurs when the difference between the actual outcome and expected outcome decreases over time. This difference, called prediction error, is represented by dopamine release by ventral tegmental area neurons. Imagine a monkey learning to associate a checker-patterned symbol with a squirt of juice (Figure 5). Initially, reward is unexpected. Receiving the unexpected juice causes an increase in firing of midbrain dopamine neurons representing a positive prediction error.64,65 Over time, the monkey learns that juice will likely follow the checker-patterned symbol, and neuronal activity shifts away from receiving the juice and toward the cue that predicts it. If the outcome is less rewarding than predicted, such as when the monkey sees the checker-patterned symbol but receives no juice, there is a decrease in neuronal firing, representing a negative prediction error.66
Figure 5.
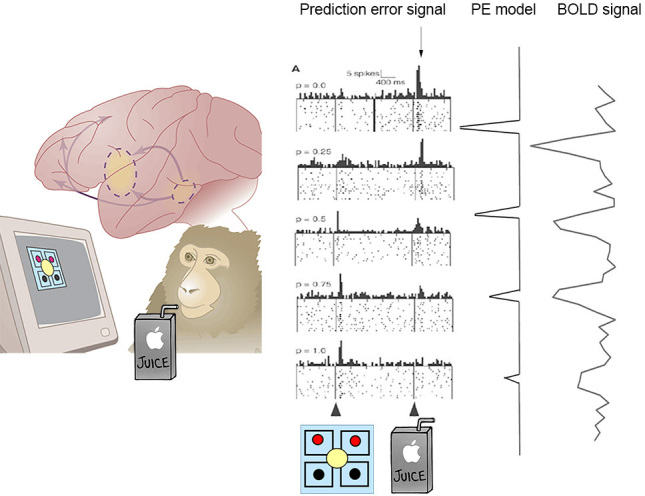
Computational fMRI model. a. An animal is learning to associate the checkered symbol with a juice reward during the recording from midbrain dopamine neurons. b. Receipt of an unexpected reward generates a prediction error (PE) signal (arrow). As the animal learns that the checkered symbol predicts reward, the PE signal shifts from receipt of reward to the stimulus predicting it. c. A computational model of PE can be generated and correlated with fMRI time series signal to produce a map of brain regions that track PE learning signals. (Adapted from Shizgal & Arvanitogiannis, Science. 2003 299(5614):1856–8. Reprinted with permission from AAAS).
The trial-to-trial prediction error signal can be computed and regressed against the time series signal during an fMRI task (Figure 5). This method known as computational fMRI produces a brain map of regions that track prediction error learning and include the ventral striatum, ventral pallidum, and medial prefrontal cortex64,67 (Figure 6). When the outcome is less rewarding than predicted, the negative prediction error signal is also tracked in the striatum.67
Figure 6.
Bilateral ventral striatum is activated during reward-based reinforcement learning in healthy controls. 6a. shows bilateral ventral putamen and 6b. shows pallidum. (From O’doherty et al. Science 2004, Reprinted with permission).
Since drugs act on the same brain systems as prediction error learning, it is reasoned that drugs could impair learning.68 The patient’s inability to pursue a long-term goal may involve her inability to accurately predict which stimuli and actions result in long-term rewards. Supporting this hypothesis, two studies have observed weaker prediction error tracking in frontal-striatal regions in patients with stimulant use disorder compared to controls.40–42,69 However, another study demonstrated an intact prediction error signal in alcohol use disorder.69 Despite some inconsistent results, this new field of computational fMRI is a model for understanding mechanisms of complex behavior.70
Cognitive control
Cognitive control processes such as planning, resolving conflict, and inhibitory control generally involve higher cortex (“top-down”) while automatic or affective processes generally involve subcortical brain regions (“bottom-up”). Addiction has been framed as excessive bottom-up and insufficient top-down processing. The disorder begins with bottom-up processing in the brainstem and subcortical reward system. Drug cue associations develop over time, evoking moods and memories that activate the hippocampus and amygdala and further strengthen bottom-up attentional bias.39,71
Active substance users have lower DLPFC activity and connectivity compared to controls,43,72 suggesting decreased top-down control. In contrast, abstinence has been associated with increased top-down resting state signal (Figure 7).45 Long-term abstainers have increased cortical synchrony in the DLPFC and decreased synchrony in subcortical networks compared to short-term abstainers.44,45 Together, these data suggest that increased top-down and decreased bottom-up signaling may be a biomarker of abstinence. This is consistent with animal experiments showing that activating top down prefrontal neurons can reduce reward-seeking behavior and suppress bottom-up fMRI signal in the striatum.73 One way to translate this intervention model from preclinical to humans is through neuromodulation.
Figure 7.
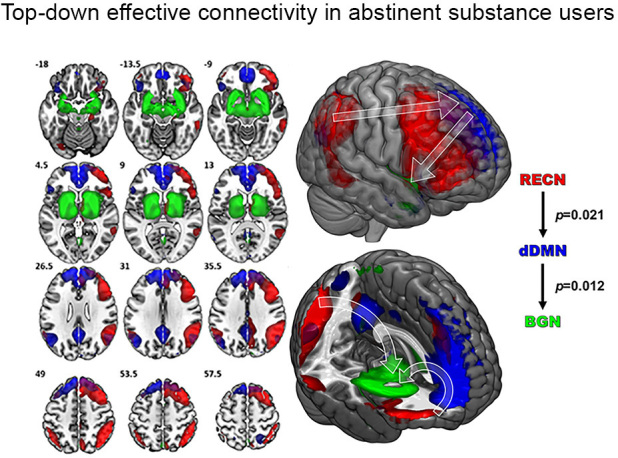
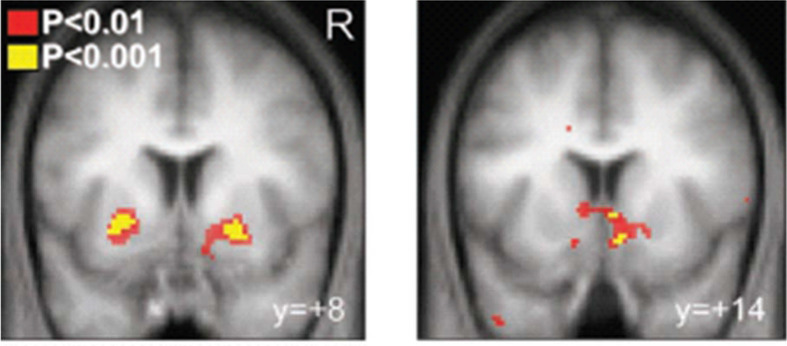
Compared to controls, long-term abstinent patients with substance use disorder (SUD) showed greater effective connectivity from the right executive control network (RECN) to the dorsal default mode network (dDMN) and from the dDMN to the basal ganglia network (BGN) , consistent with increased top-down flow of information (from Regner et al. 2016).45
neuromodulation to treat SUD
Transcranial magnetic stimulation (TMS) is a non-invasive neuromodulation technique that uses a locally applied magnetic field to induce brain activity. TMS can be either excitatory (>10 Hz) or inhibitory (<3 Hz) depending on the stimulation parameters.74 TMS is FDA-approved for treatment-refractory major depression and is beginning to make inroads into SUD, with the majority of work in nicotine use disorder.75–78 Excitatory TMS to the DLPFC led to lower cigarette consumption within the first week and at 6 months after treatment.76 It has been shown to reduce drug-craving, although in some studies cigarette consumption was unaffected79 (for review, see46,80). Currently, targeting the DLPFC is not based on patient anatomy but rather the “5 cm rule”, an empiric method of placing the TMS probe five centimeters anterior to the motor cortex. An area for improvement is better targeting of TMS energy deposition using neuro-navigation.81 With a better understanding of the anatomic substrate of craving and SUD, image-guided TMS may play a bigger role in future treatments.
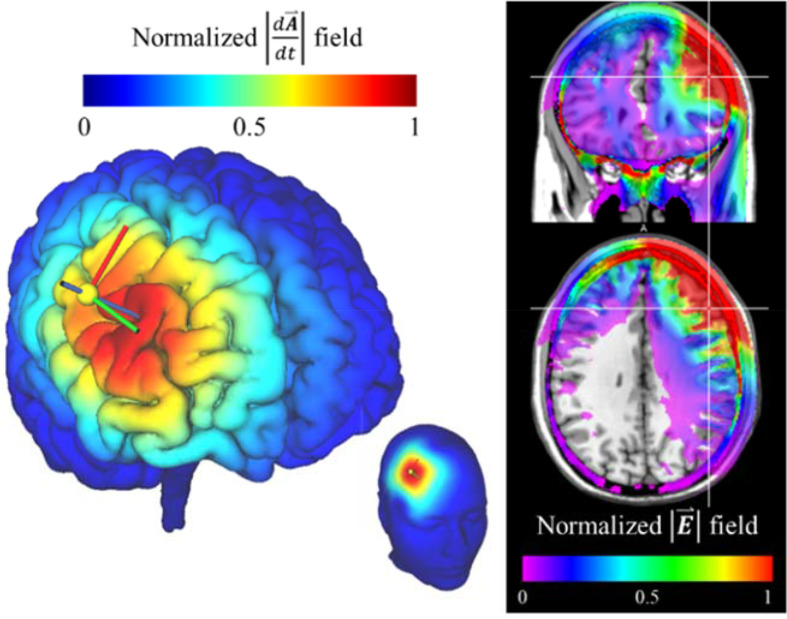
A limitation of TMS is that it primarily affects the outer layers of the brain. Stronger stimulation can reach deeper regions but is more diffuse and less targeted.82 This trade-off between electric field depth and focality limits the stimulation of deep cortical regions such as the anterior cingulate cortex and insula and precludes the TMS coil from stimulating deep nuclei (Figure 8).
Figure 8.
Model of electric field from TMS coil targeting the right dorsolateral prefrontal cortex. Predicted current flux density (left) and normalized absolute value of the electric field (right) shows the pattern of energy deposition. Maximum energy is deposited in superficial soft tissues and cerebrospinal fluid due to high tissue conductivities. Models were created using SimNIBS.
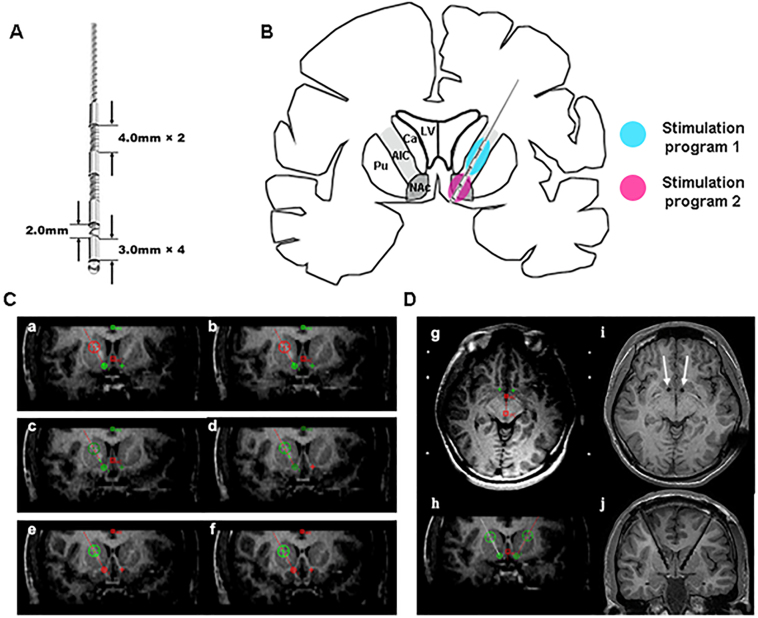
Deep brain stimulation (DBS) has been proposed as another method of neuromodulation to treat SUDs. DBS is routinely utilized to treat patients with Parkinson’s disease. Under an FDA humanitarian device exemption, DBS to the ventral striatum is used to treat obsessive compulsive disorder. Researchers working with such patients have noted the incidental remission of substance use following DBS treatment.83–85 This is consistent with animal studies showing that deep brain stimulation alters drug reinforcement86 and reinstatement of drug self-administration.87 Case reports and small series suggest that DBS has beneficial effects in heroin,88–91 cocaine,92 amphetamine93 and alcohol use disorders by reducing craving94,95 and increasing cognitive control96 (Table 2). All of these studies targeted bilateral NAc and some have targeted both the NAc and the anterior limb of the internal capsule (ALIC) simultaneously90,93 (Figure 9). However, many questions remain. The ideal location and stimulation parameters are unknown and randomized controlled trials of this intervention are lacking. Given the high placebo response rate in psychiatric conditions, well-designed trials are needed to make firmer conclusions about the potential utility of DBS neuromodulation. Should such trials continue to report encouraging results, there is no doubt that imaging will play an important role.
Table 2.
Summary of papers on DBS targets in substance and alcohol use disorders
| Ref | Author | Date | Journal | No subs | Target | Primary dx/secondary dx | Prim outcome | Result |
| 84 | Kuhn et al. | 2007 | J Neur Neurosurg Psychiatry | 1 | B.NAc | GAD, MDD/AUD | No change in anxiety | “rapid, drastic ↓ EtOH use” “EtOH free in two mos” Effects at 12 months |
| 94 | Müller et al. | 2009 | Pharmacopsych | 3 | B.NAc | AUD | OCDS, AUQ | ↓ OCD 3/3, ↓ AUQ 3/3, abstinent 1/3, ↓ drinking 2/3, ↓ craving 2/3 |
| 85 | Mantione et al. | 2010 | Neurosurgery | 1 | B.Nac | OCD, NUD, obesity | Weight, smoke, YBOCS | Quit smoking, ↓ weight, ↓YBOCS, ↓ anxiety |
| 89 | Zhou et al. | 2011 | Biol Psych | 1 | B.NAc | Heroin | Urine tox, naloxone challenge | ↓ drug use x 6 years ;↓ cigarettes |
| 88 | Valencia-Alfonso et al. | 2012 | Biol Psych | 1 | B.NAc/ALIC | Heroin | Not stated | 1 × 14 day relapse in 6m of Rx |
| 95 | Voges et al. | 2013 | World Neurosurg | 5 | Nac | AUD | OCDS, Craving, abstinence, LFP | Abstinent 2/5, ↓ craving 5/5 |
| 91 | Kuhn et al. | 2014 | Mol Psych | 2 | NAc | Opioid/amphet, AUD | Craving | ↓ Craving; ↓ heroin; in one patient, transient in anxiety, drug use, mood that responded to IPG replacement |
| 93 | Ge et al. | 2018 | World Neurosurg | 2 | B.NAc/ALIC | Methamphetamine | Urine tox, craving | Abstinent 1/2, No effect 1/2 |
| 90 | Chen et al. | 2019 | Brain Stim | 8 | B.NAc/ALIC | Heroin | Abstinence, craving, Y-BOCS, VAS | 5/8 abstinent 3 years, 2/5 relapse, one lost, ↓craving, I FDG frontal temporal |
ALIC = anterior limb internal capsule, AUD = Alcohol use disorder, AUQ = alcohol urge questionnaire, B. NAc = bilateral nucleus accumbens, BZD = benzodiazepine, GAD = generalized anxiety disorder, IPG = implanted generator, LFP = local field potential, OCD = obsessive-compulsive disorder, OCDS = obsessive-compulsive drinking scale, NUD = nicotine use disorder, YBOCS = Yale-Brown Obsessive compulsive scale
Figure 9.
Deep brain stimulation with electrodes designed for simultaneous stimulation of the NAc and ALIC. A. Customized electrode consisting of 4 stimulation contacts. B: Schematic drawing of the implanted electrode locations. The electrode can deliver different stimulation parameters to NAc and the ALIC. C: Example trajctory of DBS implanted through the ALIC into the NAc using Leksell frame and 3T MR scanner. NAc = green or red crosses; ALIC = large green or red circles with crosses. D: The trajectories of electrodes (white arrows in i, black electrodes in j) using 1.5T MR scanner. Ca = head of the caudate nucleus, ALIC = anterior limb of the internal capsule, LV = lateral ventricle, NAc = nucleus accumbens, Pu = putamen (Reprinted from Chen et al. 2019 with permission from Elsevier)90
challenges for neuroimaging in substance use research
Neuroscience has revealed many drug induced neural adaptations, but identifying the important ones is difficult. More precise cataloguing of neuroplastic events relative to the temporal stages of addiction is needed.25,97 For example, dopamine in the NAc is critical for reward and sensitization, but appears to be less important in the reinstatement of cocaine-seeking in animals. In humans, neuroimaging studies must similarly attempt to catalogue neural changes according to the phase of addiction while controlling for confounds. SUD is highly comorbid with other mental illnesses and rarely limited to a single substance. These confounding factors must be taken into account when evaluating the validity and generalizability of neuroimaging results.
The biggest challenge for fMRI studies are reproducibility and generalizability. Low statistical power in fMRI studies is well recognized and mitigated in recent years by increasing sample size and using stricter statistical analyses.98–100 Yet neuroimaging research is still prone to liberal inference, circular analyses, and reporting of misleading effect sizes.100
Conclusion
Advanced MRI and PET have been the primary tools used to study the brain’s role in substance use disorders. These methods have confirmed hypotheses that drugs and drug cues activate the mesolimbic reward and other systems. PET studies have shown that dopamine receptors and transmission are reduced in chronic substance users. As SUD is heterogeneous and complex, however, the exact role for anterior cingulate, prefrontal cortex, striatum, and insula need to be better defined in relation to drug and non-drug reward, craving, learning, and self-control. Finally, differences in neural function observed with imaging may help pinpoint targets for neuromodulation therapies, which are being investigated as treatment adjuncts. The potential of such novel treatments has generated excitement among clinicians, researchers, and patients. Given these promising findings, advances in neuroimaging research will continue to improve our understanding to stem the devastation and cost of substance use disorder.
Contributor Information
Jody Tanabe, Email: jody.tanabe@ucdenver.edu.
Michael Regner, Email: michael.regner@ucdenver.edu.
Joseph Sakai, Email: joseph.sakai@ucdenver.edu.
Diana Martinez, Email: dm437@cumc.columbia.edu.
Joshua Gowin, Email: joshua.gowin@ucdenver.edu.
REFERENCES
- 1. World Health Organization Alcohol . 2018. Available from: https://www.who.int/newsroom/fact-sheets/detail/alcohol [Sept 21].
- 2. Johnston LD, Miech RA, Malley PMO, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use: 1975-2017: Overview, key findings on adolescent drug use. Ann Arbor: The British Institute of Radiology.; 2018. [Google Scholar]
- 3. American Psychiatric A Diagnostic and statistical manual of mental disorders (DSM-5®): American psychiatric PUB. 2013. [DOI] [PubMed]
- 4. Beyers JM, Toumbourou JW, Catalano RF, Arthur MW, Hawkins JD. A cross-national comparison of risk and protective factors for adolescent substance use: the United States and Australia. J Adolesc Health 2004; 35: 3–16. doi: 10.1016/j.jadohealth.2003.08.015 [DOI] [PubMed] [Google Scholar]
- 5. Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull 1992; 112: 64–105. doi: 10.1037/0033-2909.112.1.64 [DOI] [PubMed] [Google Scholar]
- 6. Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Within-family environmental transmission of drug abuse: a Swedish national study. JAMA Psychiatry 2013; 70: 235–42. doi: 10.1001/jamapsychiatry.2013.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samhsa Risk and protective factors. 2018.
- 8. Scaramella LV, Keyes AW. The social contextual approach and rural adolescent substance use: implications for prevention in rural settings. Clin Child Fam Psychol Rev 2001; 4: 231–51. doi: 10.1023/A:1017599031343 [DOI] [PubMed] [Google Scholar]
- 9. Stone AL, Becker LG, Huber AM, Catalano RF. Review of risk and protective factors of substance use and problem use in emerging adulthood. Addict Behav 2012; 37: 747–75. doi: 10.1016/j.addbeh.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 10. von Sydow K, Lieb R, Pfister H, Höfler M, Wittchen H-U. What predicts incident use of cannabis and progression to abuse and dependence? a 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug Alcohol Depend 2002; 68: 49–64. [DOI] [PubMed] [Google Scholar]
- 11. Conger R. The social context of substance abuse: a developmental perspective : Robertson E. B, Sloboda Z, Boyd G. M, Beatty L, Kozel N. J, Rural substance abuse: state of knowledge and issues. NIDA research monograph. 168. Rockville MD: The British Institute of Radiology.; 1997. 6–36. [PubMed] [Google Scholar]
- 12. Compton WM, Thomas YF, Stinson FS, Grant BF, Prevalence GBF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the National epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 2007; 64: 566–76. doi: 10.1001/archpsyc.64.5.566 [DOI] [PubMed] [Google Scholar]
- 13. Hasin DS, Stinson FS, Ogburn E, Grant BF, Prevalence GBF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 2007; 64: 830–42. doi: 10.1001/archpsyc.64.7.830 [DOI] [PubMed] [Google Scholar]
- 14. Barrio P, Reynolds J, García-Altés A, Gual A, Anderson P. Social costs of illegal drugs, alcohol and tobacco in the European Union: a systematic review. Drug Alcohol Rev 2017; 36: 578–88. doi: 10.1111/dar.12504 [DOI] [PubMed] [Google Scholar]
- 15. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004; 291: 1238–45. doi: 10.1001/jama.291.10.1238 [DOI] [PubMed] [Google Scholar]
- 16. Collaborators GBDA Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of Disease Study. Lancet 2018; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35: 217–38. doi: 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 1988; 85: 5274–8. doi: 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 1992; 13: 177–84. doi: 10.1016/0165-6147(92)90060-J [DOI] [PubMed] [Google Scholar]
- 20. O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci 1992; 654(1 The Neurobiol): 400–15. doi: 10.1111/j.1749-6632.1992.tb25984.x [DOI] [PubMed] [Google Scholar]
- 21. Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 2001; 411: 583–7. doi: 10.1038/35079077 [DOI] [PubMed] [Google Scholar]
- 22. Sweatt JD. Neural plasticity and behavior - sixty years of conceptual advances. J Neurochem 2016; 139 Suppl 2(Suppl 2): 179–99. doi: 10.1111/jnc.13580 [DOI] [PubMed] [Google Scholar]
- 23. de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 1981; 75: 134–43. doi: 10.1007/BF00432175 [DOI] [PubMed] [Google Scholar]
- 24. Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2001; 2: 119–28. doi: 10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- 25. Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci 2013; 15: 431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol 2016; 67: 23–50. doi: 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- 27. Porrino LJ, Smith HR, Nader MA, Beveridge TJR. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 1593–600. doi: 10.1016/j.pnpbp.2007.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. . Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 1997; 386: 827–30. doi: 10.1038/386827a0 [DOI] [PubMed] [Google Scholar]
- 29. Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. . Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 1998; 155: 1009–15. doi: 10.1176/ajp.155.8.1009 [DOI] [PubMed] [Google Scholar]
- 30. Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. . Acute effects of cocaine on human brain activity and emotion. Neuron 1997; 19: 591–611. doi: 10.1016/S0896-6273(00)80374-8 [DOI] [PubMed] [Google Scholar]
- 31. Luijten M, Schellekens AF, Kühn S, Machielse MWJ, Sescousse G. Disruption of Reward Processing in Addiction : An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry 2017; 74: 387–98. doi: 10.1001/jamapsychiatry.2016.3084 [DOI] [PubMed] [Google Scholar]
- 32. Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D, et al. . Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse 2010; 64: 397–402. doi: 10.1002/syn.20741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci 2011; 33: 1318–26. doi: 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- 34. Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. . Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage 2012; 60: 252–62. doi: 10.1016/j.neuroimage.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. . Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 2000; 157: 1789–98. doi: 10.1176/appi.ajp.157.11.1789 [DOI] [PubMed] [Google Scholar]
- 36. Kober H, Lacadie CM, Wexler BE, Malison RT, Sinha R, Potenza MN. Brain activity during cocaine craving and gambling urges: an fMRI study. Neuropsychopharmacology 2016; 41: 628–37. doi: 10.1038/npp.2015.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, et al. . Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 2001; 158: 86–95. doi: 10.1176/appi.ajp.158.1.86 [DOI] [PubMed] [Google Scholar]
- 38. Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend 2011; 115(1-2): 137–44. doi: 10.1016/j.drugalcdep.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry 1999; 156: 11–18. doi: 10.1176/ajp.156.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rose EJ, Salmeron BJ, Ross TJ, Waltz J, Schweitzer JB, McClure SM, et al. . Temporal difference error prediction signal dysregulation in cocaine dependence. Neuropsychopharmacology 2014; 39: 1732–42. doi: 10.1038/npp.2014.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanabe J, Reynolds J, Krmpotich T, Claus E, Thompson LL, Du YP, et al. . Reduced neural tracking of prediction error in substance-dependent individuals. Am J Psychiatry 2013; 170: 1356–63. doi: 10.1176/appi.ajp.2013.12091257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang JM, Zhu L, Brown VM, De La Garza R, Newton T, King-Casas B, et al. . In cocaine dependence, neural prediction errors during loss avoidance are increased with cocaine deprivation and predict drug use. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4: 291-299. doi: 10.1016/j.bpsc.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in "bottom-up" and "top-down" neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage 2011; 56: 2258–75. doi: 10.1016/j.neuroimage.2011.03.054 [DOI] [PubMed] [Google Scholar]
- 44. Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcohol Clin Exp Res 2013; 37: 75–85. doi: 10.1111/j.1530-0277.2012.01859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Regner MF, Saenz N, Maharajh K, Yamamoto DJ, Mohl B, Wylie K, et al. . Top-down network effective connectivity in abstinent substance dependent individuals. PLoS One 2016; 11: e0164818: e0164818. doi: 10.1371/journal.pone.0164818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, et al. . What goes up, can come down: novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res 2015; 1628(Pt A): 199–209 1628(Pt A). doi: 10.1016/j.brainres.2015.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. . Consensus Nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–9. doi: 10.1038/sj.jcbfm.9600493 [DOI] [PubMed] [Google Scholar]
- 48. Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000; 20: 423–51. doi: 10.1097/00004647-200003000-00001 [DOI] [PubMed] [Google Scholar]
- 49. Asensio S, Morales JL, Senabre I, Romero MJ, Beltran MA, Flores-Bellver M, et al. . Magnetic resonance imaging structural alterations in brain of alcohol abusers and its association with impulsivity. Addict Biol 2016; 21: 962–71. doi: 10.1111/adb.12257 [DOI] [PubMed] [Google Scholar]
- 50. Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, Pan P. Regional gray matter deficits in alcohol dependence: a meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend 2015; 153: 22–8. doi: 10.1016/j.drugalcdep.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 51. Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One 2013; 8: e59645 10.1371/journal.pone.0059645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Daumann J, Koester P, Becker B, Wagner D, Imperati D, Gouzoulis-Mayfrank E, et al. . Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometry. Neuroimage 2011; 54: 794–801. doi: 10.1016/j.neuroimage.2010.08.065 [DOI] [PubMed] [Google Scholar]
- 53. Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev 2013; 37: 300–16. doi: 10.1016/j.neubiorev.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, et al. . Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry 2009; 65: 160–4. doi: 10.1016/j.biopsych.2008.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pan P, Shi H, Zhong J, Xiao P, Shen Y, Wu L, et al. . Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci 2013; 34: 813–7. doi: 10.1007/s10072-012-1256-x [DOI] [PubMed] [Google Scholar]
- 56. Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. . Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 2004; 55: 77–84. doi: 10.1016/S0006-3223(03)00610-3 [DOI] [PubMed] [Google Scholar]
- 57. Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry 2005; 57: 967–74. doi: 10.1016/j.biopsych.2005.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, et al. . Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 2005; 162: 1461–72. doi: 10.1176/appi.ajp.162.8.1461 [DOI] [PubMed] [Google Scholar]
- 59. Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol 2013; 23: 615–24. doi: 10.1016/j.conb.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 60. Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA. Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse 2009; 63: 681–9. doi: 10.1002/syn.20646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. . Imaging dopamine transmission in cocaine dependence: response to treatment linked to neurochemistry. Am J Psychiatry 2011; 168: 634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, et al. . Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 2007; 27: 12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005; 8: 1481–9. doi: 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- 64. O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron 2003; 38: 329–37. doi: 10.1016/S0896-6273(03)00169-7 [DOI] [PubMed] [Google Scholar]
- 65. Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 1997; 275: 1593–9. doi: 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- 66. Schultz W. Dopamine reward prediction error coding. Dialogues Clin Neurosci 2016; 18: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron 2003; 38: 339–46. doi: 10.1016/S0896-6273(03)00154-5 [DOI] [PubMed] [Google Scholar]
- 68. Redish AD. Addiction as a computational process gone awry. Science 2004; 306: 1944–7. doi: 10.1126/science.1102384 [DOI] [PubMed] [Google Scholar]
- 69. Huys QJM, Deserno L, Obermayer K, Schlagenhauf F, Heinz A. Model-free Temporal-Difference learning and dopamine in alcohol dependence: examining concepts from theory and animals in human imaging. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1: 401–10. doi: 10.1016/j.bpsc.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 70. Paulus MP, Huys QJM, Maia TV. A roadmap for the development of applied computational psychiatry. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1: 386–92. doi: 10.1016/j.bpsc.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Volkow N, Dependence D. Drug dependence and addiction, III: expectation and brain function in drug abuse. Am J Psychiatry 2004; 161: 621: 621. doi: 10.1176/appi.ajp.161.4.621 [DOI] [PubMed] [Google Scholar]
- 72. Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology 2014; 39: 2288–98. doi: 10.1038/npp.2014.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Deisseroth K, Optogenetics DK. Optogenetics.. Nat Methods 2011; 8: 26. doi: 10.1038/nmeth.f.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118. doi: 10.1016/j.brs.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 75. Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009; 104: 653–60. doi: 10.1111/j.1360-0443.2008.02448.x [DOI] [PubMed] [Google Scholar]
- 76. Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, et al. . Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry 2014; 76: 742–9. doi: 10.1016/j.biopsych.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 77. Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, et al. . Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 2013; 73: 714–20. doi: 10.1016/j.biopsych.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul 2014; 7: 226–33. doi: 10.1016/j.brs.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 79. Wing VC, Bacher I, Wu BS, Daskalakis ZJ, George TP. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res 2012; 139(1-3): 264–6. doi: 10.1016/j.schres.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 80. Salling MC, Martinez D. Brain stimulation in addiction. Neuropsychopharmacology 2016; 41: 2798–809. doi: 10.1038/npp.2016.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, et al. . Prefrontal rTMS for treating depression: location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimul 2013; 6: 108–17. doi: 10.1016/j.brs.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Deng Z-D, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul 2013; 6: 1–13. doi: 10.1016/j.brs.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH, et al. . Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res 2009; 15: 196–201. doi: 10.1159/000228930 [DOI] [PubMed] [Google Scholar]
- 84. Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, et al. . Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry 2007; 78: 1152–3. doi: 10.1136/jnnp.2006.113092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mantione M, van de Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery 2010; 66: E218 E218;-discussion E.. doi: 10.1227/01.NEU.0000360570.40339.64 [DOI] [PubMed] [Google Scholar]
- 86. Liu H-Y, Jin J, Tang J-S, Sun W-X, Jia H, Yang X-P, et al. . Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol 2008; 13: 40–6. doi: 10.1111/j.1369-1600.2007.00088.x [DOI] [PubMed] [Google Scholar]
- 87. Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. . Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci 2008; 28: 8735–9. doi: 10.1523/JNEUROSCI.5277-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Valencia-Alfonso C-E, Luigjes J, Smolders R, Cohen MX, Levar N, Mazaheri A, et al. . Effective deep brain stimulation in heroin addiction: a case report with complementary intracranial electroencephalogram. Biol Psychiatry 2012; 71: e35–7. doi: 10.1016/j.biopsych.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 89. Zhou H, Xu J, Jiang J. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: a case report. Biol Psychiatry 2011; 69: e41–2. doi: 10.1016/j.biopsych.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 90. Chen L, Li N, Ge S, Lozano AM, Lee DJ, Yang C, et al. . Long-term results after deep brain stimulation of nucleus accumbens and the anterior limb of the internal capsule for preventing heroin relapse: an open-label pilot study. Brain Stimul 2019; 12: 175–83. doi: 10.1016/j.brs.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 91. Kuhn J, Möller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TOJ, et al. . Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry 2014; 19: 145–6. doi: 10.1038/mp.2012.196 [DOI] [PubMed] [Google Scholar]
- 92. Gonçalves-Ferreira A, do Couto FS, Rainha Campos A, Lucas Neto LP, Gonçalves-Ferreira D, Teixeira J. Deep brain stimulation for refractory cocaine dependence. Biol Psychiatry 2016; 79: e87–9. doi: 10.1016/j.biopsych.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 93. Ge S, Chen Y, Li N, Qu L, Li Y, Jing J, Qu L, Wang X, et al. . Deep brain stimulation of nucleus accumbens for methamphetamine addiction: two case reports. World Neurosurg 2019; 122: 512-517. doi: 10.1016/j.wneu.2018.11.056 [DOI] [PubMed] [Google Scholar]
- 94. Müller UJ, Sturm V, Voges J, Heinze H-J, Galazky I, Büntjen L, et al. . Nucleus Accumbens Deep Brain Stimulation for Alcohol Addiction - Safety and Clinical Long-term Results of a Pilot Trial. Pharmacopsychiatry 2016; 49: 170–3. doi: 10.1055/s-0042-104507 [DOI] [PubMed] [Google Scholar]
- 95. Voges J, Müller U, Bogerts B, Münte T, Heinze H-J. Deep brain stimulation surgery for alcohol addiction. World Neurosurg 2013; 80(3-4): S28.e21–S28.e31. doi: 10.1016/j.wneu.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 96. Kuhn J, Gründler TOJ, Bauer R, Huff W, Fischer AG, Lenartz D, et al. . Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol 2011; 16: 620–3. doi: 10.1111/j.1369-1600.2011.00337.x [DOI] [PubMed] [Google Scholar]
- 97. Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nat Neurosci 2005; 8: 1440–1. doi: 10.1038/nn1105-1440 [DOI] [PubMed] [Google Scholar]
- 98. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 2016; 113: 7900–5. doi: 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mumford JA, Nichols TE. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage 2008; 39: 261–8. doi: 10.1016/j.neuroimage.2007.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. . Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017; 18: 115–26. doi: 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]