Table 1. Optimization of the Reaction Conditionsa.
| entry | electrode | electrolyte | current (mA) | additive | time (h) | yield (%)b |
|---|---|---|---|---|---|---|
| 1 | C/C | LiClO4 | 10 | 3 | 60 | |
| 2c | C/C | LiClO4 | 10 | 3 | 52 | |
| 3 | C/C | LiClO4 | 10 | AcOH | 3 | 43 |
| 4 | C/C | LiClO4 | 10 | TsOH | 3 | 77 |
| 5 | C/C | LiClO4 | 10 | TsOH | 4.5 | 72 |
| 6 | C/C | LiClO4 | 10 | TsOH | 2.5 | 79 |
| 7 | C/C | LiClO4 | 10 | TsOH | 2 | 74 |
| 8 | C/C | LiClO4 | 5 | TsOH | 4.5 | 78 |
| 9 | C/C | LiClO4 | 15 | TsOH | 2 | 74 |
| 10 | C/Pt | LiClO4 | 10 | TsOH | 2.5 | 66 |
| 11 | C/Ni | LiClO4 | 10 | TsOH | 2.5 | 51 |
| 12 | C/Cu | LiClO4 | 10 | TsOH | 2.5 | 32 |
| 13 | C/Fe | LiClO4 | 10 | TsOH | 2.5 | 31 |
| 14 | C/C | Bu4NBF4 | 10 | TsOH | 2.5 | 63 |
| 15 | C/C | 10 | TsOH | 2.5 | 82 | |
| 16 | C/C | 10 | 2.5 | 53 | ||
| 17d | C/C | 10 | TsOH | 2.5 | 67 | |
| 18e | C/C | 10 | TsOH | 2.5 | 68 | |
| 19f | C/C | 10 | TsOH | 2.5 | 0 |
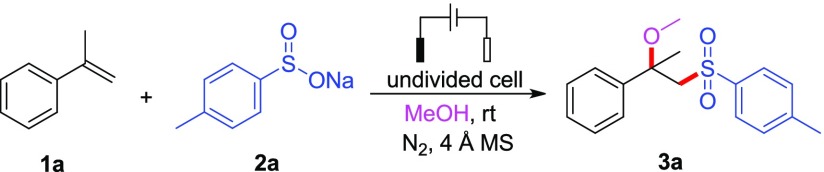
Reaction conditions: 1a (0.5 mmol), 2a (2.0 equiv), MeOH (8 mL), electrolyte (0.5 mmol), additive (0.5 mmol), 4 Å MS (200 mg), undivided cell, room temperature, and nitrogen atmosphere.
Yields based on 1a.
Under air without 4 Å MS.
2a (1.5 equiv).
2a (3.0 equiv).
Without electric current.