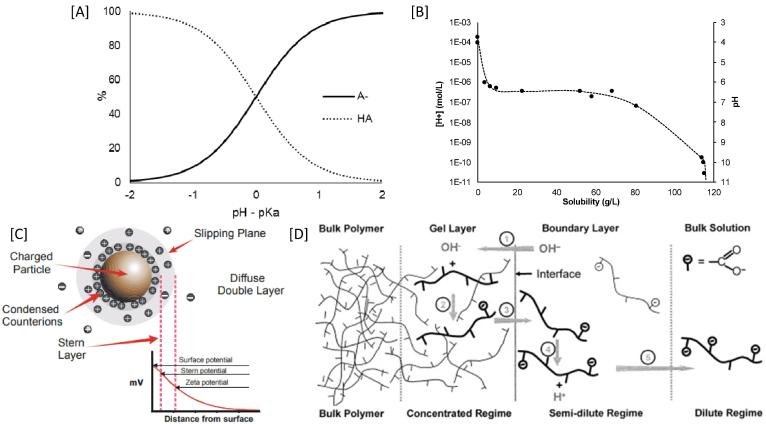
Fig. 2.
[A] pH-dependant ionisation of a weak acid [HA] and its conjugated base [A-] drawn using Henderson-Hasselbalch equation; [B] Ionisation and solubility of a pH-responsive polymer as a function of pH (redrawn using data from (Nguyen and Fogler, 2005); [C] A schematic showing the potential difference as a function of distance from the charged surface of a particle in a medium (Malvern, 2017); [D] Dissolution mechanism of pH-responsive polymers reproduced with permission from (Nguyen and Fogler, 2005). The encircled numbers in [D] represent (1) Diffusion of water and hydroxyl ions into the polymer matrix to form a gel layer, (2) Ionization of polymer chains in the gel layer, (3) Disentanglement of polymer chains out of the gel layer to the polymer-solution interface, (4) Further ionization of polymer chains at the polymer interface, (5) Diffusion of disentangled polymer chains away from the interface toward the bulk solution.