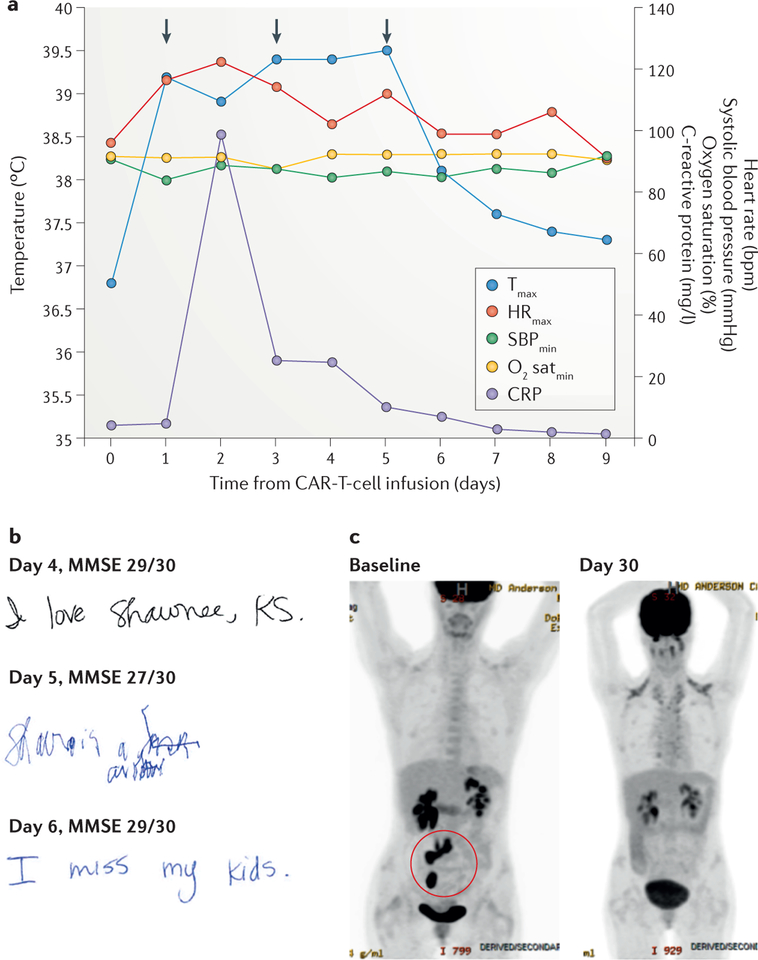
Figure 1 |. Clinical case study.
The findings of key clinical assessments are shown for a representative patient with cytokine-release syndrome and chimeric antigen receptor (CAR)-T-cell-related encephalopathy syndrome after anti-CD19 CAR-T-cell therapy for refractory diffuse large-B-cell lymphoma. a | The graph shows the patient’s maximum temperature (Tmax), maximum heart rate (HRmax), minimum systolic blood pressure (SBPmin), minimum oxygen saturation (O2 satmin), and serum C-reactive protein (CRP) level recorded on each day after anti-CD19 CAR-T-cell therapy. The anti-IL-6 receptor antibody tocilizumab was administered on days 1, 3, and 5 (arrows) for the treatment of hypotension, hypoxia, and encephalopathy, respectively. b | Handwriting samples and mini mental status exam (MMSE) scores obtained on days 4, 5, and 6 after CAR-T-cell therapy; note how the patient’s handwriting was markedly impaired on day 5, despite only a small decrease in their MMSE score. c | 2-[18F]fluoro-2-deoxy-d-glucose PET images showing the retroperitoneal lymph nodes and ileocolic region harbouring lymphoma at baseline (highlighted in red circle; bottom left), and loss of tracer uptake indicative of induction of disease remission at 30 days after infusion of CAR T cells (bottom right).