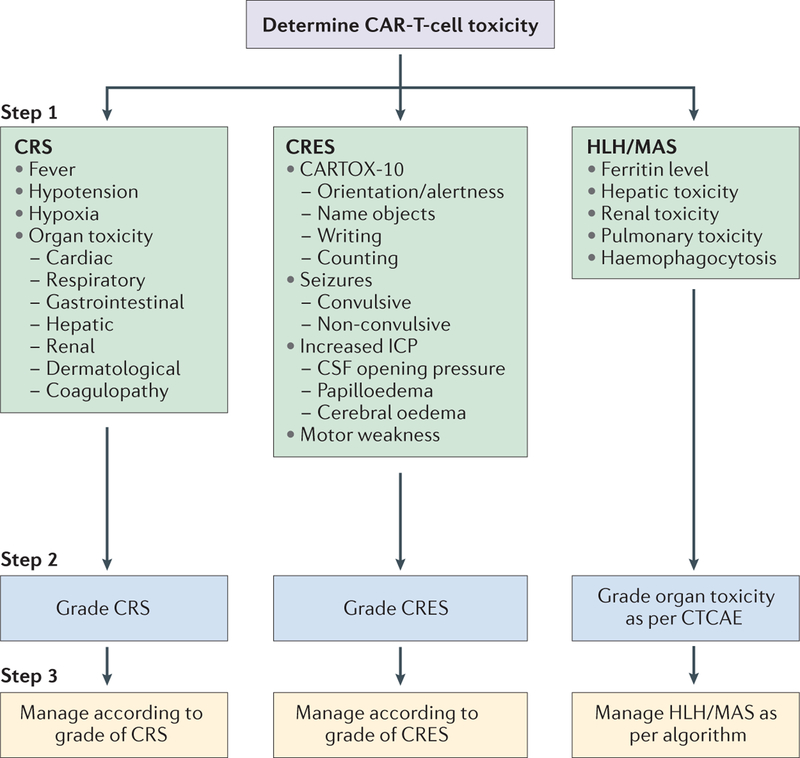
Figure 2 |. Three-step approach to the assessment and management of acute toxicities associated with chimeric antigen receptor (CAR)-T-cell therapy.
Step 1: the patient’s clinical and biological symptoms should be monitored to determine the nature of the CAR-T-cell-related toxicity, in order to diagnose cytokine-release syndrome (CRS), CAR-T-cell-related encephalopathy syndrome (CRES), and haemophagocytic lymphohistiocytosis/macrophage-activation syndrome (HLH/MAS; BOX 5). Step 2: the severity of CRS, CRES, and HLH/MAS should be graded using the criteria provided in TABLE 2, TABLE 4, and the Common Terminology Criteria for Adverse Events, version 4.03 (CTCAE)43, respectively. Step 3: the toxicities should be treated according to the management algorithms we have provided for CRS (TABLE 3), CRES (BOX 2), and HLH/MAS (FIG. 3). CARTOX-10, CAR-T-cell-therapy-associated toxicity 10-point neurological assessment; CSF, cerebrospinal fluid; ICP, intracranial pressure.