Figure 7.
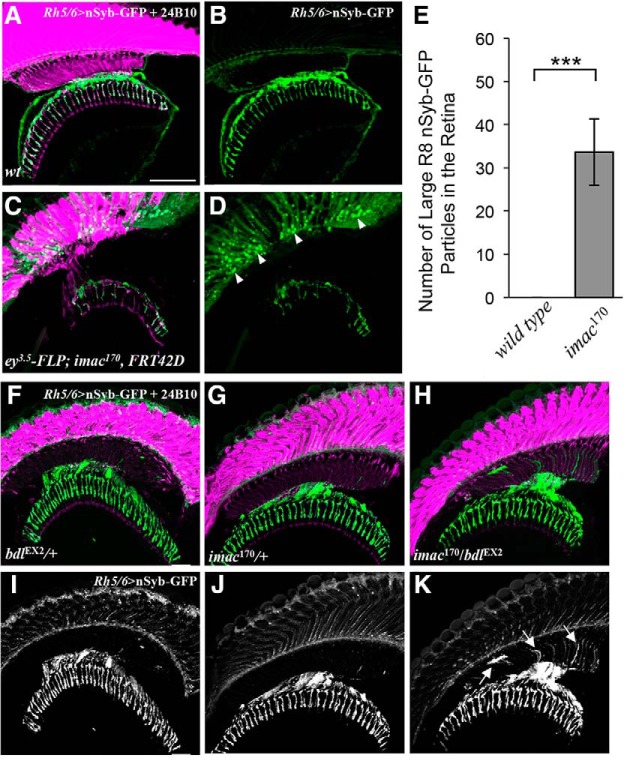
bdl interacts genetically with imac in the control of SV transport in R8 axons. A–D, Loss of imac caused a failure of SV components to transport from R8 cell bodies into axons. Frozen sections of adult heads expressing UAS-nSybGFP under control of the green-sensitive-R8-specific driver Rh6-GAL4, were stained with anti-GFP (green) and MAb24B10 (magenta). A, In wild-type (n = 6 animals), nSyb-GFP staining was predominantly localized to R8 axonal terminals in the medulla. B, The section in A was visualized with nSyb-GFP staining only. C, In eye-specific large imac170 homozygous clones (n = 6 animals), the levels of nSyb-GFP staining in R8 axons were greatly reduced. A large number of nSyb-GFP-positive large aggregates were observed in R8 cell bodies in the retina. D, The section in C was visualized with nSyb-GFP staining only. E, The number of abnormal large nSyb-GFP-positive aggregates in the retina was quantified. Loss of imac greatly increased the number of nSyb-GFP-positive aggregates in the retina. Student's t test, ***p = 0.0073. Error Bars indicate SEM. F–K, Frozen sections of adult heads expressing UAS-n-Syb-GFP under control of the R8-specific driver Rh5/6-GAL4, were stained with anti-GFP (green) and MAb24B10 (magenta). In most bdlEX2/+ (F, I; 14 of 15 animals) or imac170/+ heterozygotes (G, J; 14 of 16 animals), nSyb-GFP staining was predominantly localized to R8 axonal terminals in the medulla region. In the majority of bdlEX2/imac170 transheterozygotes (12 of 15 animals), however, strong n-Syb-GFP staining was also observed in the proximal portion of R8 axons in the lamina. Arrows indicate proximal portions of R8 axons with mislocalized nSyb-GFP. Scale bar, 20 μm.