Abstract
Objective
Research has shown that analyzing intrusion errors generated on verbal learning and memory measures is helpful for distinguishing between the memory disorders associated with Alzheimer’s disease (AD) and other neurological disorders, including Huntington’s disease (HD). Moreover, preliminary evidence suggests that certain clinical populations may be prone to exhibit different types of intrusion errors.
Method
We examined the prevalence of two new California Verbal Learning Test-3 (CVLT-3) intrusion subtypes – across-trial novel intrusions and across/within trial repeated intrusions – in individuals with AD or HD. We hypothesized that the encoding/storage impairment associated with medial-temporal involvement in AD would result in a greater number of novel intrusions on the delayed recall trials of the CVLT-3, whereas the executive dysfunction associated with subcortical-frontal involvement in HD would result in a greater number of repeated intrusions across trials.
Results
The AD group generated significantly more across-trial novel intrusions than across/within trial repeated intrusions on the delayed cued-recall trials, whereas the HD group showed the opposite pattern on the delayed free-recall trials.
Conclusions
These new intrusion subtypes, combined with traditional memory analyses (e.g., recall versus recognition performance), promise to enhance our ability to distinguish between the memory disorders associated with primarily medial-temporal versus subcortical-frontal involvement.
Keywords: Alzheimer disease, Huntington disease, memory disorders, verbal learning, memory, memory and learning tests, neuropsychological tests
Research has shown that the number of intrusion errors generated on verbal memory tests often differs across various neurological populations. In particular, the total intrusions on a recall task tends to be significantly higher in patients with medial-temporal and/or dorsomedial thalamic involvement compared to individuals with subcortical-frontal involvement (Butters et al., 1987; Delis et al., 1991; Helkala et al., 1989; Kramer et al., 1988; Lafosse et al., 1997; Libon et al., 1997). For example, Delis et al. (1991) found that patients with mild Alzheimer’s disease (AD) or Korsakoff’s syndrome (KS) generated significantly more intrusion errors than patients with mild Huntington’s disease (HD). Nonetheless, there are some limitations in relying on the total number of intrusion errors to distinguish between the memory disorders associated with different neurological conditions. First, some studies have reported comparable numbers of total intrusions in patients whose initial neuropathology often involves primarily medial-temporal versus subcortical-frontal involvement (Kramer et al., 1989; Rouleau et al., 2001). For example, Kramer et al. (1989) found that AD and HD patients did not differ significantly in the total number of intrusions generated on the original California Verbal Learning Test (CVLT; Delis et al., 1987). Second, the total number of intrusions may tend to differ in AD versus HD in group studies, but on an individual case basis, one occasionally sees an AD patient who generates only a few intrusions, or a patient with subcortical-frontal involvement (e.g., HD) who generates exceptionally high numbers of intrusions (Delis et al., 2005).
In a recent review of memory disorders, which included an important study by Davis et al., (2002), Delis et al. (2017) reported a shortcoming in how existing clinical memory tests, including the original CVLT and CVLT-II, analyze intrusion responses. Davis et al. (2002) and Delis et al. (2017) both noted that, on existing clinical measures, intrusion errors are analyzed with respect to the particular type of recall trial on which they are generated (e.g., free- versus cued-recall). In some cases, these trial-specific analyses have clinical utility; for example, the analysis of cued-recall intrusions on the CVLT has been shown to enhance the distinction between the memory profiles of AD versus other neurological disorders (Delis et al., 1991; Delis et al., 2000; Hamilton et al., 2004; Massman et al., 1992). In other cases, however, trial-specific intrusion analyses (e.g., whether an intrusion response is novel or repeated within a particular recall trial) have not shown clinical utility in distinguishing between different memory profiles. A possible reason for this shortcoming is that this latter analysis focuses on whether an intrusion response is novel or repeated only within a single recall trial and does not consider whether an intrusion response is repeated across the various recall trials of the test (Davis et al, 2002). However, as discussed by Delis et al. (2017), patients with initial involvement primarily in medial-temporal versus subcortical-frontal regions often differ in the nature of the intrusion errors they generate across the various learning and recall trials of the same test.
Individuals with primarily subcortical-frontal involvement (e.g., HD) may report an intrusion response on an early learning trial and repeat that response across later learning and recall trials due to source memory problems (i.e., did the response come from the examiner or examinee?; Delis et al., 2017). In contrast, individuals with primarily medial-temporal involvement (e.g., AD) are prone to generate more novel than repeated intrusions, particularly on the delayed cued-recall trials when the category cues tend to elicit confabulatory responses, because their severe encoding/storage impairment diminishes their ability to encode any information into memory, including any intrusion responses that they may have generated on earlier learning trials (Delis et al., 1991; Delis et al., 2017).
In the current study, we examined the utility of two new CVLT-3 intrusion subtypes – across-trial novel intrusions versus across/within trial repeated intrusions – in individuals with AD or HD. The focus of these intrusion analyses was on the delayed-recall trials, given that (a) many intrusions generated on the immediate-recall trials will, by definition, be novel, and (b) intrusion rates tend to be more prevalent on the delayed cued-recall trials. Consistent with the different mechanisms of memory impairment attributed to AD and HD, our hypothesis was that the AD group would generate significantly more across-trial novel intrusions than across/within trial repeated intrusions, whereas the HD group would show the opposite pattern.
Method
Study participants included 22 individuals with AD and 22 individuals with HD. Individuals with AD were recruited from the Shiley-Marcos Alzheimer’s Disease Research Center (ADRC) affiliated with the University of California, San Diego (UCSD). Diagnoses of individuals with probable AD were made by a senior staff neurologist at the ADRC and were consistent with the criteria established by the National Institute of Aging–Alzheimer’s Association (NIA–AA) workgroup (McKhann et al., 1984; McKhann et al., 2011). Individuals with HD were recruited from the Huntington’s Disease Clinical Research Center (HDCRC) at UCSD and were administered the Unified Huntington’s Disease Rating Scale (UHDRS; Huntington Study Group, 1996) by a senior staff neurologist. Individuals with HD were diagnosed with definite HD on the basis of unequivocal motor signs on the UHDRS and a positive family history of HD. In addition, all HD participants had a CAG repeat length greater than 39 (range = 40-52, M = 44.95, SD = 3.54), indicating that all carried the fully penetrant genetic mutation for HD. Exclusionary criteria for study participants included any major neurological, psychiatric, or other medical illness aside from AD or HD diagnosis. The Dementia Rating Scale (DRS; Mattis, 1988) or the DRS-2 was administered to all participants to provide an assessment of global cognitive function. The study was completed in accordance with the Helsinki Declaration. All participants provided informed written consent and the study was approved by the Institutional Review Board of UCSD.
The CVLT-II was administered using standard procedures outlined by Delis and colleagues (2000). Given that the CVLT-II and CVLT-3 contain identical target words on the recall trials, CVLT-3 coding procedures were applied to CVLT-II data to generate scores for two new CVLT-3 intrusion subtypes: across-trial novel intrusions (any intrusion that has not been reported by the examinee on any previous trial, including the List B trial; Delis et al., 2017), and across/within trial repeated intrusions (any intrusion that has been reported at least once by the examinee on any of the previous trials and/or within the same trial; Delis et al., 2017).
Analyses were conducted in the Statistical Package for the Social Sciences Version 25. Prior to conducting the analyses, one-way analysis of variance (ANOVA) tests and chi-square analyses were conducted to examine group differences on demographic variables, including age, gender, education, and DRS/DRS-2 scores. Additionally, preliminary ANOVA and ANCOVA tests were conducted to determine whether demographic variables or DRS/DRS-2 scores were significant predictors of intrusion errors. ANCOVA tests with repeated measures were conducted to examine the effects of group, intrusion subtype, and a group x intrusion subtype interaction on intrusions summed across (a) the two delayed cued-recall trials, and (b) the two delayed free-recall trials. In the context of significant group x intrusion subtype interaction effects, simple effects analyses were conducted to examine differences in intrusion subtypes within each group as well as group differences at each level of intrusion subtype. Effect size values associated with significant within (r; Morris & DeShon, 2002) and between (Cohen’s d) group differences were calculated and reported.
Results
Demographic information and descriptive statistics on intrusion measures for the AD and HD groups are provided in Table 1. As expected, the AD group was significantly older than the HD group, F(1,42)=145.03, p<.001. Additionally, the AD group completed significantly more years of education than the HD group, F(1,42)=6.17, p<.05. The AD group contained a higher proportion of men than women, whereas the HD group contained a higher proportion of women than men, χ2(1,N=44)=4.46, p<.05. DRS/DRS-2 scores were comparable between the AD and HD groups, F(1,42)=0.002, p>.05.
Table 1.
Demographic information and descriptive statistics on intrusion measures for the Alzheimer’s (AD) and Huntington’s (HD) disease groups.
| Variable | AD | HD | ||
|---|---|---|---|---|
| Demographics | ||||
| n | 22 | 22 | ||
| % Female | 31.82 | 63.64 | ||
| M (SD) | Range | M (SD) | Range | |
| Age | 81.05 (7.67) | 71-95 | 48.64 (10.03) | 25-61 |
| Education | 15.91 (2.71) | 11-20 | 14.09 (2.11) | 12-18 |
| DRS/DRS-2 Total Score | 124.82 (3.70) | 118-129 | 124.86 (3.72) | 118-129 |
| CVLT-3 Trials 1-5 Total | 22.91 (6.58) | 12-34 | 29.77 (9.52) | 9-47 |
| Delayed Cued-Recall Intrusions | ||||
| Across-Trial Novel | 8.68 (7.11) | 0-24 | 1.73 (2.93) | 0-14 |
| Across/Within Trial Repeated | 4.91 (7.10) | 0-31 | 2.45 (3.04) | 0-11 |
| Total | 13.59 (13.16) | 0-55 | 4.18 (5.52) | 0-25 |
| Delayed Free-Recall Intrusions | ||||
| Across-Trial Novel | 0.41 (0.73) | 0-3 | 0.64 (1.05) | 0-3 |
| Across/Within Trial Repeated | 0.41 (0.67) | 0-2 | 1.14 (1.42) | 0-5 |
| Total | 0.82 (1.01) | 0-3 | 1.77 (1.90) | 0-6 |
Note: AD = Alzheimer’s disease; HD = Huntington’s disease; M = mean; SD = standard deviation; DRS = Dementia Rating Scale; CVLT = California Verbal Learning Test.
Age was a significant predictor of intrusion errors and was therefore included as a covariate in analyses, F(1,42)=5.87, p=.02. Gender predicted intrusion errors at a trend level and was therefore controlled for in analyses, F(1,42)=3.87, p=.06. Neither education, F(1,42)=0.87, p=.36, nor DRS/DRS-2 scores, F(1,42)=2.09, p=.16, were significant predictors of intrusion errors; these variables were excluded from analyses.
There was a significant group x intrusion subtype interaction effect on the delayed cued-recall trials, F(1,40)=4.22, p<.05. On the delayed cued-recall trials, the AD group made significantly more across-trial novel intrusions than across/within trial repeated intrusions (p=.006; r=.70). In contrast, the HD group had a comparable number of novel and repeated intrusions (p>.05). Additionally, the AD group made significantly more across-trial novel intrusions than the HD group (p=.03; d=1.28). There were no group differences on across/within trial repeated intrusions (p=.54). There were no main effects of group F(1,40)=2.29, p=.14, or intrusion subtype, F(1,40)=0.94, p=.34, on the delayed cued-recall trials. The prevalence of intrusion subtypes in AD versus HD on the delayed cued-recall trials is illustrated in Figure 1.
Figure 1.
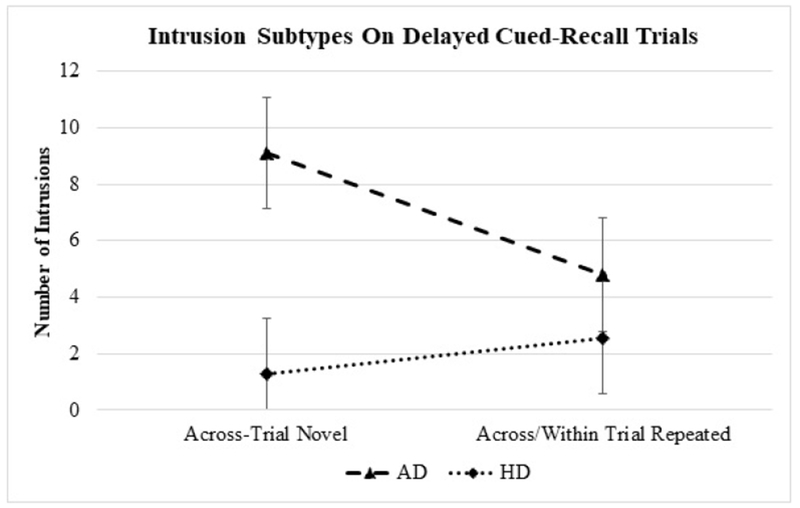
Prevalence of intrusion subtypes (estimated marginal means with standard errors) in AD versus HD on the delayed cued-recall trials.
There was also a significant group x intrusion subtype interaction effect on the delayed free-recall trials, F(1,40)=4.68, p=.04. On the delayed free-recall trials, the HD group made significantly more across/within trial repeated intrusions than across-trial novel intrusions (p=.02); this difference (although smaller in magnitude than the mean difference in intrusion subtypes on delayed cued-recall observed within the AD group; refer to Table 1), was associated with a medium effect size (r=.31). The AD group had a comparable number of across-trial novel and across/within trial repeated intrusions (p>.05) on the delayed free-recall trails. There were no group differences on novel or repeated intrusions (ps>.05). There were no main effects of group F(1,40)=0.64, p=.43, or intrusion subtype, F(1,40)=1.93, p=.17, on the delayed free-recall trials.
Although not a primary focus of the present study, we wish to note that the AD and HD groups did not differ in the total number of intrusions generated across all recall trials, F(1,40)=1.37, p=.25.
Discussion
In the present study, we examined the prevalence and pattern of two new CVLT-3 intrusion measures – across-trial novel intrusions and across/within trial repeated intrusions – in individuals with AD or HD. The findings indicated that 1) on the delayed cued-recall trials, the AD group generated significantly more across-trial novel intrusions than across/within trial repeated intrusions, and they made significantly more across-trial novel intrusions than the HD group, and 2) on the delayed free-recall trials, the HD group generated significantly more across/within trial repeated intrusions than across-trial novel intrusions. Thus, building on the early work by Davis et al. (2002), the present results illustrate the clinical utility of new intrusion analyses that, when combined with other, established memory parameters (e.g., recall versus recognition memory), promise to enhance our ability to distinguish between the memory disorders associated with primarily medial-temporal versus subcortical-frontal involvement.
The question arises as to whether these new intrusion analyses enhance our assessment of memory disorders relative to traditional intrusion measures. First, in the current sample, the AD and HD groups did not differ in the total number of intrusions generated across all trials. Second, previous studies using the original CVLT and the CVLT-II showed that the analysis of cued-recall intrusions was helpful in distinguishing between the memory profiles of AD versus HD (Delis et al., 1991; Delis et al., 2000; Massman et al., 1992). An exploratory logistic regression analysis in the present study indicated that when accounting for across-trial novel intrusions on the delayed cued-recall trials, total intrusions (either on the delayed cued-recall trials or across all recall trials) did not significantly predict AD versus HD group membership. Moreover, an exploratory correlation analysis indicated that across/within trial repeated intrusions on the delayed-free recall trials were significantly correlated with overall learning (Trials 1-5 Total, p = .018), but no other significant correlations between intrusion subtypes and overall learning were observed. This is not surprising, given previous evidence against an association between intrusion errors and traditional recall measures on the CVLT in patients with AD or HD (Delis et al., 2003). These findings suggest that the inclusion of across-trial novel versus repeated intrusion analyses may provide additional insight into the pattern of memory deficits associated with AD and HD, over and above examining traditional intrusion measures. Given that intrusion errors predict progression from normal cognition to mild cognitive impairment (MCI) and mild AD dementia (Bondi et al., 1999; Thomas et al., 2018a,b), and given that intrusion rates are higher in MCI individuals with the amnestic subtype compared to the dysexecutive or mixed subtypes (Libon et al., 2011), these new CVLT-3 intrusion subtypes may demonstrate diagnostic utility among individuals in preclinical stages of neurodegenerative disease as well.
Delis et al. (2017) proposed possible mechanisms for why such differences in the nature of across-trial intrusions may occur between individuals with initial primary involvement in medial-temporal versus subcortical-frontal regions. Individuals with primarily subcortical-frontal involvement (e.g., HD) often generate at least some intrusions on the immediate-recall trials of word-list memory tests (Baldo et al., 2002). Across the learning trials, examinees repeatedly hear the target words, which in individuals with frontal involvement, may pull for semantically-related intrusions due to disinhibition stemming from executive dysfunction. That is, the presentation of a word automatically activates the semantic network associated with that word, and individuals with frontal involvement may have difficulty inhibiting the generation of at least some of those semantic associations. As a result, these individuals are prone to generate intrusions on the immediate-recall trials (Baldo et al., 2002). Importantly, once an individual with primarily frontal involvement reports an intrusion, that response may fall prey to another aspect of executive dysfunction associated with frontal involvement: source memory problems. That is, after the individual reports an intrusion, he or she may have difficulty remembering the source of that response (i.e., the examiner versus examinee). As a result, an intrusion response generated on an earlier learning trial by an individual with primarily frontal involvement may be repeated by that individual across the remaining recall trials of the test. Moreover, individuals with primarily frontal involvement are prone to repeat intrusions within the same trial, which could also increase their total number of intrusion errors.
A different mechanism may underlie the generation of across-trial intrusions in individuals with severe encoding/storage deficits associated with primarily medial-temporal involvement, such as those with AD (Delis et al., 2017). The tendency of AD patients to exhibit high rates of intrusions seems to go beyond basic disinhibition to reflect a profound encoding/storage deficit coupled with better albeit declining language and semantic processing skills (Delis et al., 2000). This cognitive profile may elicit confabulatory tendencies, especially on cued-recall trials when the category cues elicit semantic associations to those categories. However, in contrast to individuals with frontal involvement, when individuals with AD report an intrusion response on an earlier trial, their severe encoding/storage deficit will likely impair their ability to encode that response into long-term memory, thereby precluding them from the opportunity to exhibit source memory problems for that response on later recall trials. These proposed mechanisms for differential intrusion subtypes in individuals with primarily medial-temporal versus subcortical-frontal involvement are in line with Davis and colleagues’ discussion of the roles of semantic knowledge and executive function deficits in intrusions generated by individuals with AD versus ischemic vascular dementia, respectively (Davis et al., 2002). Of course, mechanisms underlying cognitive deficits are rarely absolute in clinical populations. Evidence suggests that although AD is the neuropathology most frequently detected in the brains of deceased older adults, it rarely occurs in isolation and is often accompanied by other neuropathologies (e.g., Lewy body disease, vascular brain injury, TDP-43, hippocampal sclerosis; Boyle et al., 2018; Brenowitz et al., 2017). Thus, executive dysfunction may play some role in the generation of intrusion errors in AD, just as HD may also be associated with at least some temporal lobe dysfunction. The present study provides evidence for heterogeneity in the underlying neuropathologies that cause dementia, as both AD and HD patients generated novel and repeated intrusions albeit to varying degrees.
The present study is not without limitations. We acknowledge that a number of demographic, genetic, behavioral (e.g., motor functioning), and psychiatric characteristics are typically used to distinguish individuals with HD from AD. However, the present findings may apply to the assessment of different memory disorders associated not only with these neurodegenerative conditions, but to other conditions involving primarily medial-temporal or subcortical-frontal involvement or of individuals in preclinical stages of neurodegenerative disease when other clinical features have not clearly manifested. Additionally, we acknowledge that the utilization of relatively small sample sizes in the present study may potentially impact the generalizability of the findings, which should be taken into consideration when interpreting the present results.
In sum, the present findings provide evidence that the encoding/storage impairment associated with AD may yield a significantly higher number of across-trial novel versus across/within trial repeated intrusions on recall, whereas the executive dysfunction (i.e., disinhibition, source memory impairment) associated with HD may result in the opposite pattern. These new CVLT-3 intrusion subtypes may enhance our ability to distinguish between the memory disorders associated with primarily medial-temporal versus subcortical-frontal involvement.
Acknowledgements
Dean C. Delis, Ph.D. is a co-author of the CVLT and receives royalties for the test. D.P.S. is a paid consultant for Aptinyx and Takeda Pharmaceuticals. M.W.B. receives royalties from Oxford University Press and is a paid consultant for Eisai, Novartis, and Roche Pharmaceuticals. There are no other conflicts of interest to be declared by any authors. This work was supported, in part, by National Institutes of Health (NIH) grants R01 AG034202 and P30 AG059299 to P.E.G., K24 AG026431 and R01 AG049810 to M.W.B., and P50 AG005131 to the Shiley-Marcos Alzheimer’s Disease Research Center, and a Huntington’s Disease Society of America Center of Excellence grant to J.C.B.
References
- Baldo JV, Delis DC, Kramer JH, & Shimamura AP (2002). Memory performance on the California Verbal Learning Test-II: Findings from patients with focal frontal lesions. Journal of the International Neuropsychological Society, 8, 539–546. doi:10.1017.S1355617701020288 [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, & Thal LJ (1999). Neuropsychological function and Apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychology and Aging, 14(2), 295–303. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, & Bennett DA (2018). Person-specific contribution of neuropathologies to cognitive loss in old age. Annals of Neurology, 83, 74–83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz WD, Keene CD, Hawes SE, Hubbard RA, Longstreth WT Jr., Woltjer RL, & Kukull WA (2017). Alzheimer’s disease neuropathologic change, Lewy body disease, and vascular brain injury in clinic- and community-based samples. Neurobiology of Aging, 53, 83–92. doi: 10.1016/j.neurobiolaging.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters N, Granholm E, Salmon DP, Grant I, & Wolfe J (1987). Episodic and semantic memory: A comparison of amnesic and demented patients. Journal of Clinical and Experimental Neuropsychology, 9(5), 479–497. doi: 10.1080/01688638708410764 [DOI] [PubMed] [Google Scholar]
- Davis KL, Price CC, Kaplan E, & Libon DJ (2002). Error analysis of the nine-word California Verbal Learning Test (CVLT-9) among older adults with and without dementia. Clinical Neuropsychology, 16(1), 81–89. doi: 10.1076/clin.16.1.81.8330 [DOI] [PubMed] [Google Scholar]
- Delis DC, Jacobson M, Bondi MW, Hamilton JM, & Salmon DP (2003). The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society, 9, 936–946. doi: 10.1017/S1355617703960139 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (1987). California Verbal Learning Test. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California Verbal Learning Test-II, Second Edition San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2017). California Verbal Learning Test-3, Third Edition San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, & Kramer JH (1991). Profiles of demented and amnesic patients on the California Verbal Learning Test: Implications for the assessment of memory disorders. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 3(1), 19–26. doi: 10.1037/1040-3590.3.1.19 [DOI] [Google Scholar]
- Delis DC, Wetter SR, Jacobson MW, Peavy G, Hamilton J, Gongvatana A, & Salmon DP (2005). Recall discriminability: Utility of a new CVLT-II measure in the differential diagnosis of dementia. Journal of the International Neuropsychological Society, 11, 708–715. doi: 10.1017/S1355617705050812 [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Delis DC, Hansen LA, Masliah E, & Thal LJ (2004). A comparison of episodic memory deficits in neuropathologically-confirmed dementia with Lewy bodies and Alzheimer’s disease. Journal of the International Neuropsychological Society, 10, 689–697. doi: 10.1017/S1355617704105043 [DOI] [PubMed] [Google Scholar]
- Helkala EL, Laulumaa V, Soininen H, & Riekkinen PJ (1989). Different error pattern of episodic and semantic memory in Alzheimer’s disease and Parkinson’s disease with dementia. Neuropsychologia, 27(10), 1241–1248. doi: 10.1016/0028-3932(89)90036-5 [DOI] [PubMed] [Google Scholar]
- Huntington Study Group. (1996). Unified Huntington’s disease rating scale: Reliability and consistency. Movement Disorders, 11, 136–142. doi: 10.1002/mds.870110204 [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Blusewicz MJ, Brandt J, Ober B, & Strauss M (1988). Verbal memory errors in Alzheimer’s and Huntington’s dementias. Developmental Neuropsychology, 4(1), 1–15. doi: 10.1080/87565648809540385 [DOI] [Google Scholar]
- Kramer JH, Levin BE, Brandt J, & Delis DC (1989). Differentiation of Alzheimer’s, Huntington’s, and Parkinson’s disease patients on the basis of verbal learning characteristics. Neuropsychology, 3(2), 111–120. doi: 10.1037/h0091766 [DOI] [Google Scholar]
- Lafosse JM, Reed BR, Mungas D, Sterling SB, Wahbeh H, & Jagust WJ (1997). Fluency and memory differences between ischemic vascular dementia and Alzheimer’s disease. Neuropsychology, 11(4), 514–522. doi: 10.1037/0894-4105.11.4.514 [DOI] [PubMed] [Google Scholar]
- Libon DJ, Bogdanoff B, Bonavita J, Skalina S, Cloud BS, Resh R, & Ball SK (1997). Dementia associated with periventricular and deep white matter alterations: A subtype of subcortical dementia. Archives of Clinical Neuropsychology, 12(3), 239–250. doi: 10.1016/S0887-6177(96)00041-8 [DOI] [PubMed] [Google Scholar]
- Libon DJ, Bondi MW, Price CC, Lamar M, Eppig J, Wambach DM, … & Penney DL (2011). Verbal serial list learning in mild cognitive impairment: A profile analysis of interference, forgetting, and errors. Journal of the International Neuropsychological Society, 17, 905–914. doi: 10.1017/S1355617711000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massman PJ, Delis DC, Butters N, Dupont RM, & Gillin JC (1992). The subcortical dysfunction hypothesis of memory deficits in depression: Neuropsychological validation in a subgroup of patients. Journal of Clinical and Experimental Neuropsychology, 14(5), 687–706. doi: 10.1080/01688639208402856 [DOI] [PubMed] [Google Scholar]
- Mattis S (1988). Dementia Rating Scale. Professional manual. Florida: Psychological Assessment Resources. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, & Stadlan M (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCD-ADRDA work group. Neurology, 34, 939–944. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, & Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroup. Alzheimer’s & Dementia, 7, 263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SB & DeShon RP (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods, 7, 105–125. doi: 10.1037/1082-989X.7.1.105 [DOI] [PubMed] [Google Scholar]
- Rouleau I, Imbault H, Lamframboise M, & Bedard MA (2001). Pattern of intrusions in verbal recall: Comparison of Alzheimer’s disease, Parkinson’s disease, and frontal lobe dementia. Brain and Cognition, 46(1-2), 244–249. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Edmonds EC, Eppig J, Salmon DP, & Bondi MW (2018). Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. Journal of Alzheimer’s Disease. doi: 10.3233/JAD-180229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Eppig J, Edmonds EC, Jacobs DM, Libon DJ, Au R, & The Alzheimer’s Disease Neuroimaging Initiative. (2018). Word-list intrusion errors predict progression to mild cognitive impairment. Neuropsychology, 32(2), 235–245. doi: 10.1037/neu0000413 [DOI] [PMC free article] [PubMed] [Google Scholar]


