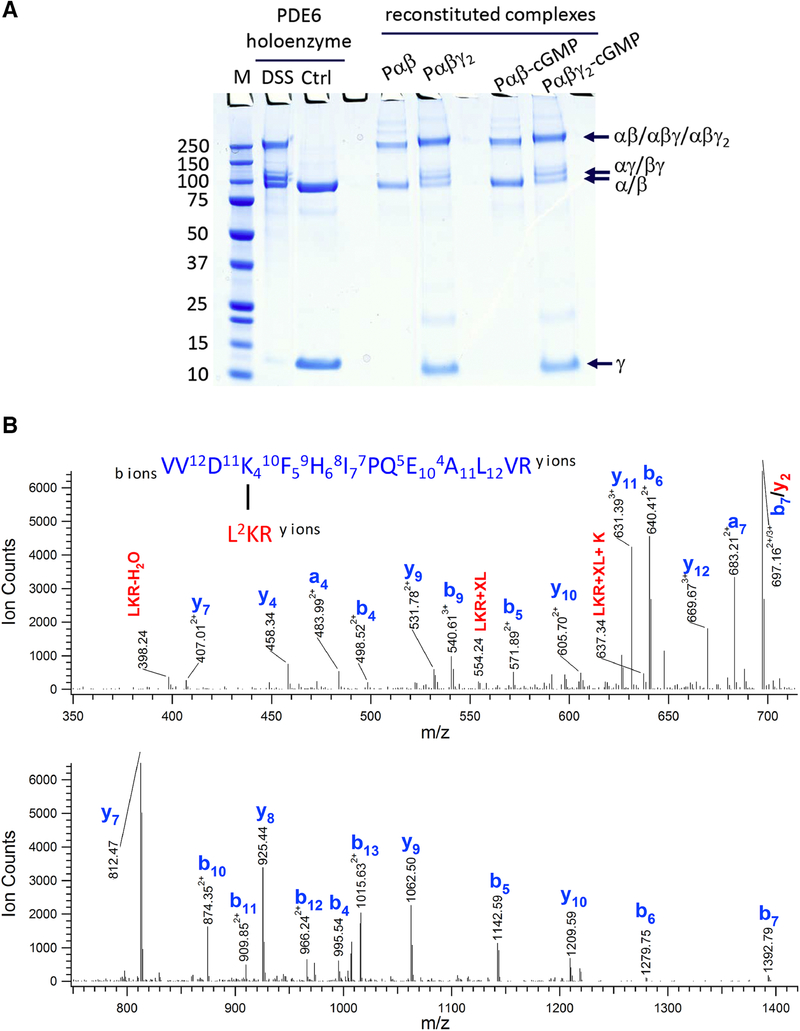
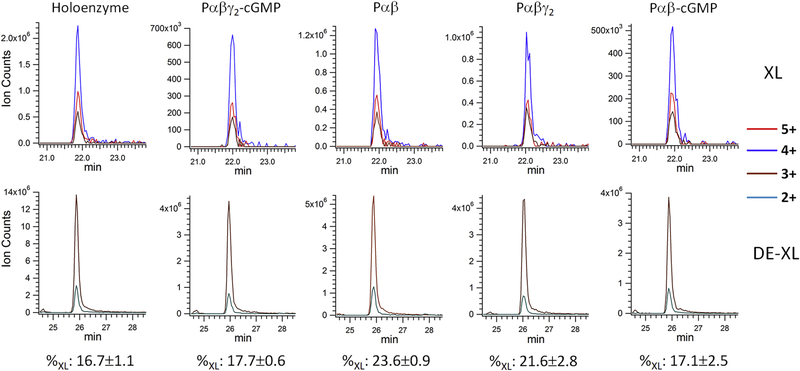
Figure 1.
Chemical cross-linking of the PDE6 complexes in various allosteric states. A) Purified PDE6 holoenzyme or the Pαβ catalytic dimer reconstituted with Pγ or/and cGMP were cross-linked with DSS in 200-fold molar excess. A control sample (Ctrl) was treated identically except that the DSS cross-linker was omitted. Gel bands of uncross-linked subunits (γ and α/β) and cross-linked subunits (αγ or βγ at ~110 kDa; αβ or αβγ or αβγ2 at ~220 kDa) are indicated. B) Tandem MS spectrum of a cross-linked peptide from the 220 kDa band, with fragments from peptide V531-R544 and L580-R582. C) Extracted ion chromatograms (XIC) of the Lys534-Lys581 cross-linked peptides (XL) in charge state +5, +4 and +3 from various PDE6 complexes were overlaid (top panel). XIC of Lys534 dead-end cross-link (DE-XL) in charge state +3 and +2 from various PDE6 complexes were overlaid (bottom panel). MS signals of XL and DE-XL were used to calculate the cross-linking percentage (%XL) of Lys534-Lys581 in various PDE6 complexes.