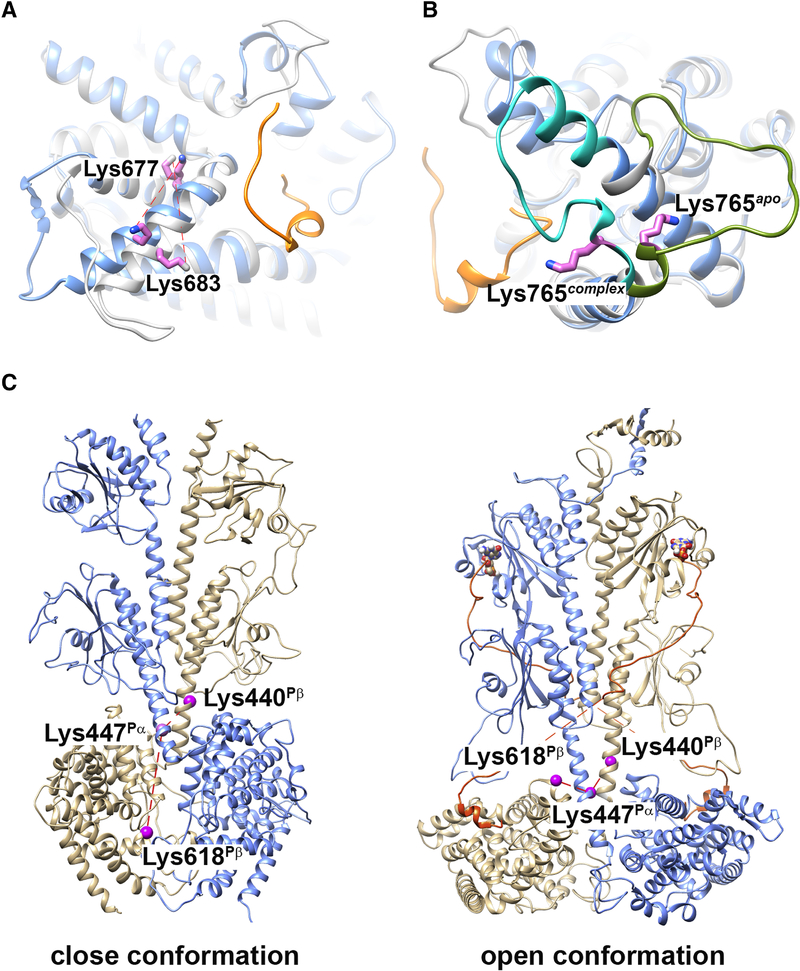
Figure 3.
Structural elements identified by cross-linking propensity of PDE6 complexes in various allosteric states. (A and B) Superimposition of comparative models for Pα catalytic domain with (blue; PDB ID: 6MZB) or without (gray; template PDB ID: 2H40) a fragment of Pγ bound. The C-terminal segment of Pγ in the PDE6 holoenzyme structure is colored in orange. Cross-linked Pα residues Lys683-Lys677 (A) and Lys765 (B) are indicated as violet sticks. C) Comparative models for Pαβ catalytic dimer without (left; template PDB ID: 3IBJ) or with (right; PDB ID: 6MZB) ligand and Pγ binding. Pα subunit is colored in blue and Pβ subunit in tan. The C-terminal segment of Pγ is colored in orange. Cross-linked Pα residues Lys447Pα, Lys440Pβ and Lys618Pβ are indicated as violet spheres.