Abstract
The circadian clock, which consists of endogenous self-sustained and cell-autonomous oscillations in mammalian cells, is known to regulate a wide range of peripheral tissues. The unique upregulation of a clock gene, neuronal PAS domain protein 2 (Npas2), observed along with fibroblast aging prompted us to investigate the role of Npas2 in the homeostasis of dermal structure using in vivo and in vitro wound healing models. Time-course healing of a full-thickness skin punched wound exhibited significantly faster wound closure in Npas2−/− mice than wild-type (WT) C57Bl/6J mice. Dorsal skin fibroblasts isolated from WT, Npas2+/−, and Npas2−/− mice exhibited consistent profiles of core clock gene expression except for Npas2 and Per2. In vitro behavioral characterizations of dermal fibroblasts revealed that Npas2−/− mutation was associated with increased proliferation, migration, and cell contraction measured by floating collagen gel contraction and single-cell force contraction assays. Npas2 knockout fibroblasts carrying sustained the high expression level of type XII and XIV FAICT collagens and synthesized dermis-like thick collagen fibers in vitro. Confocal laser scanning microscopy demonstrated the reconstruction of dermis-like collagen architecture in the wound healing area of Npas2−/− mice. This study indicates that the induced Npas2 expression in fibroblasts may interfere with skin homeostasis, wound healing, and dermal tissue reconstruction, providing a basis for novel therapeutic target and strategy.
Keywords: Npas2, fibroblast, wound healing, collagen, circadian rhythm
INTRODUCTION
The circadian rhythm, known as endogenous self-sustained and cell-autonomous oscillations of 24 hr rhythms in mammalian cells, is responsible for a wide range of physiological homeostasis functions (Franzoni et al., 2017), and the disruption of this rhythm is involved in chronic diseases, such as cardiovascular disease, diabetes, metabolic and sleep disorders, infertility, and impaired wound healing (Miller et al., 2004; Turek et al., 2005; Oishi et al., 2006; Wijnen and Young, 2006; O’Neil et al., 2013; Sipahi et al., 2014). A previous study reported that the database of human burn injuries showed that wounds injured during the night (the rest period) healed more slowly than wounds acquired during the day (the active period) (Hoyle et al., 2017). Those results suggest a regulatory role of circadian rhythm in wound healing, albeit the mechanism of how the circadian rhythm contributes to skin wound healing is still unclear.
Circadian rhythm is regulated in the central brain by the suprachiasmatic nuclei (SCN) of the hypothalamus, which is the circadian pacemaker (Akhtar et al., 2002; O’Neil et al., 2013). One of the circadian rhythm core regulators, neuronal PAS domain protein2 (NPAS2) is a member of the basic helix-loop-helix (bHLH)-PAS family of transcription factors and is a paralog of the circadian locomotor output cycles kaput (CLOCK). NPAS2 or CLOCK dimerizes with brain and muscle Arnt-like protein-1 (BMAL1) to regulate the gene transcription of two other circadian gene clusters; period (PER) and cryptochrome (CRY). PER and CRY then suppress the expression of NAPS2, CLOCK, and BMAL1 by a transcription/translation feedback loop system (Vitaterna et al., 1994; Bunger et al., 2000). Previous studies have revealed that Npas2 expression occurs in the mammalian forebrain and central brain but not in the SCN. However, the distinct expression of Npas2 was reported in peripheral tissue, including the heart, liver, vasculature, and skin (Zhou et al., 1997; McNamara et al., 2001; Gilles-Gonzalez and Gonzalez, 2004; Yamamoto et al., 2004; Bertolucci et al., 2008).
Mouse skin fibroblasts have been reported to express Npas2, which might compensate for the lack of Clock expression (Landgraf et al., 2016). NPAS2 was identified among significantly upregulated genes in aging human skin by microarray analysis (Glass et al., 2013). Taken together, we have hypothesized that Npas2 in skin fibroblasts plays a key role in homeostatic maintenance, and therefore is a key factor during skin wound healing. The objective of our study was to address this hypothesis using Npas2 knockout mice.
MATERIALS AND METHODS
Animal Ethics Statement
The Npas2 knockout (KO) mice (B6.129S6-Npas2tm1Slm/ J,Jackson Laboratory, Bar Harbor, ME) of the c57B1/6J background were used in this experiment. Npas2 heterozygous mutant (Npas2+/−) mice were generated from cryopreserved sperm samples, and an active breeding colony was established at UCLA. Both Npas2−/− and Npas2+/− mice were used as the experimental groups, and C57Bl/6J wild-type (WT) mice were used as the control group. All of the experimental protocols using animals were reviewed and approved by the UCLA animal research committee (ARC# 2003–009) and followed the Public Health Service Policy for the Humane Care and Use of Laboratory Animals and the UCLA Animal Care and Use Training Manual guidelines. All of the animals had free access to food and water and were maintained in regular housing with a 12 hr light/dark cycle at the Division of Laboratory Animal Medicine, UCLA.
Mouse Dorsal Skin Full-Thickness Excisional Wound Model
The 9- to 14-week-old mice weighing approximately 25 g (WT: four males and four females, Npas2+/−: seven males and Npas2 −/−: seven males) were used for the dorsal skin full-thickness wound experiment. After anesthesia with isoflurane inhalation, identical skin wounds were created on the right and left sides of dorsal skin simultaneously by punching a full-thickness skin wound, passing though the panniculus carnosus layer, with a 5 mm dermal biopsy punch (INTEGRA, Integra Life Sciences, Plainsboro, Nj). These surgeries were performed between 11 a.m. and 1 p. m. Standardized photographs during the course of wound healing were obtained at 0, 2, 4, 6, and 12 days. The skin wound area was measured at each time point (NIH ImageJ ver.1.51). The wound areas on each day were compared by the Kruskal-Wallis test with Dunn’s post-test. These mice were sacrificed at 7 days (n = 4 in each group) and 14 days (WT: four mice, Npas2+/−: three mice, and Npas2−/−: three mice) for histological analysis. The dorsal skin containing the wound area was dissected as a 1 cm square and immediately fixed with 10% neutral buffered formalin. The sections were stained with hematoxylin and eosin (H-E) for histological evaluation.
Dermal Fibroblast Cell Culture
Primary fibroblasts from the mouse dorsal skin of each of the three genotypes were cultured using an explant method. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and 100 U penicillin/0.1 mg/mL streptomycin at 37°C, 5% CO2 in a humidified incubator. Their genotype was determined by polymerase chain reaction (PCR) targeting WT and mutant Npas2 gene alleles.
WST-1 Cell Proliferation Assay
The cell proliferation assay was performed using WST-1 reagent (Roche Applied Science, Indianapolis, IN). A total of 2,000 cells were seeded into a 96-well reading plate and cultured for the predetermined time points (Days 1, 3, 5, and 7). At each time point, the culture medium was changed to 10% WST-1 regent with medium and incubated for 3 hr (n = 4 per time point). The absorption value was read in a spectrophotometer at 450 nm with a plate reader (SYHNERGY h1 plate reader, Biotek, Winooski, VT) and compared by two-way analysis of variance (ANOVA), followed by the Tukey test at each time point.
Circadian Gene Expression in Skin Fibroblasts
Steady-state mRNA expression levels of eight core circadian genes in skin fibroblasts were determined by quantitative real-time PCR (RT-PCR) using Taqman MGB probes (Thermo Fisher Scientific Inc., Waltham, MA). Fibroblasts were cultured in 24-well plates and synchronized at 80% to 90% confluency by adding 100 nM dexa- methasone to the medium and incubating for 2 hr, followed by washing with DMEM (Nagoshi et al., 2004). Total RNA was extracted using an RNeasy kit (Qiagen, Valencia, CA) every 6 hr, starting at 0 −48 hr (n = 4 per time point) after the synchronization, and their quality and quantity were confirmed by NanoDrop (Thermo Fisher Scientific Inc.). The RT-PCR was performed using commercially available primer/probe mixes (Thermo Fisher Scientific Inc.) as follows: Npas2 (Mm01239312_m1), Bmal1 (= Arntl: Mm00500223_m1), Clock (Mm00455950_m1), Per1 (Mm0050 1813_m1), Per2 (Mm00478099_m1), Per3 (Mm00478120_m1), Cry1 (Mm00514392_m1), and Cry2 (Mm01331539_m1). Gapdh was used as an internal control. In addition, the LacZ reporter gene expression was determined. The statistical analysis was performed first by two-way ANOVA. The group with the significant interaction P value (P < 0.05) by two-way ANOVA and the gene expression at each time point was further subjected to the Tukey test.
In Vitro Wound Healing Scratch Plate Assay
Fibroblasts were seeded into a 6-well plate and were synchronized as above. After 2 hr, scratch lines were created with a 20 μL plastic pipette and were washed with medium (n = 5 per group). These scratched areas were captured by time-lapse photomicrography every hour from 0 to 24 hr. The number of migrated cells into the scratched area was counted at 12 and 24 hr and compared by one-way ANOVA with post hoc Holm test.
Floating Collagen Gel Contraction Assay
The floating collagen gel contraction assay was performed following the previously established protocol with some modifications (Ngo et al., 2006). A 500 μL aliquot of collagen gel mixture (Collagen Type I, Corning, Manassas, VA) containing fibroblasts (50,000 cells) was applied to a 24-well plate (n = 5 in each group) and placed at room temperature for 20 min. The solidified gels were transferred to a 100 mm diameter dish and cultured (37°C, 5% CO2 in a humidified incubator). The gel images were scanned by a scanner at 0, 6, 12, 24, 48, and 72 hr. The collagen gel area at each time point was measured (NIH ImageJ ver.1.51) and compared by two-way ANOVA, followed by the Tukey test at each time point.
Single-Cell Contraction Assessment
Single-cell contraction was measured using fluorescently labeled elastomeric contractible surfaces (FLECS) (Forcyte Biotechnologies Inc., Los Angeles, CA) (Koziol-White et al., 2016). FLECS plates with the soft silicone elastomer filmed bottom were micropatterned with fluorescent fibrinogen in uniform “X” shapes (70 μm diagonal by 10 μm thick). Approximately 30,000 cells were seeded into a well of 24-well
FLECS plate. The plates were placed at room temperature for 40 min and in an incubator (37°C, 5% CO2) for 30 min for cell attachment. After incubation for initial cell attachment to the X-shape pattern, floating cells were removed by washing with medium, and the plates were incubated for an additional 8 hr. Nuclear staining was performed with Hoechst 33,342 (1:10,000). The images of the fluorescent fibrinogen on the X-shape patterns were captured using a fluorescence microscope with a rhodamine filter. For single-cell contraction evaluation, micropatterns associated with a single nucleus attached at the center of the X shape were selected and categorized to either the no-contract or contract group by comparison with the no-cell pattern. The ratio of contracted patterns per captured image (containing approximately 1,000 X-shape patterns) was compared among each genotype (n = 5). The statistical analysis was performed by one-way ANOVA with the post hoc Holm test.
Gene Expression for Actin, Integrin, and Collagen Subunits
Total RNA samples were extracted from fibroblasts every 6 hr, from 24 to 48 hr after synchronization, as described above. The RNA samples were used for evaluating the gene expression of actin subunits ―β-actin (Actb: Mm02619580_g1) and α-smooth muscle actin (α-SMA, Acta2: Mm00725412_s1) (Fig. 3G); integrin subunits—integrin αV (ItgaV: Mm00434486_m1), integrin β3 (Itgb3: Mm00443980_m1), and integrin β5 (Itgb5: Mm00439825_m1) (Fig. 3H); and collagen subunits—type I (Col1a1: Mm00801666_g1 and Col1a2: Mm00483888_m1), type III (Col3a1: Mm00802300_m1), type XII (Col12a1: Mm01148576_m1) and type XIV (Col14a1: Mm008052 69_m1) by Taqman-based qRT-PCR (Fig. 4A). The statistical analysis was performed by two-way ANOVA and Tukey test at each time point.
Fig. 3.
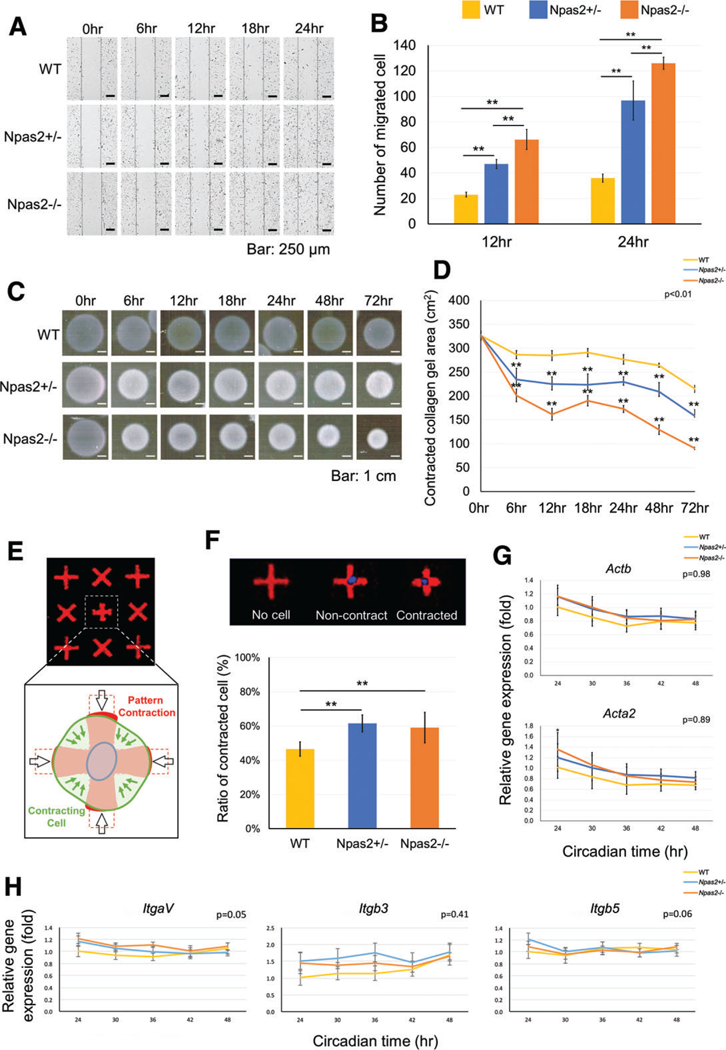
In vitro wound healing experiment using WT, Npas2+/− and Npas2−/− fibroblasts. (A) Images of time-lapse micrographs captured the progressive scratch wound healing assay. The number of migrated cells within the scratched area was significantly larger in the Npas2 KO groups at 6 hr and 12 hr (**P < 0.01). (C) Standardized images of floating collagen gel depicted an increased collagen gel contraction in the Npas2 KO fibroblast groups. (D) The area of collagen gels decreased over time. The gel contraction speed was faster in Npas2 KO fibroblasts (**P < 0.01, significant difference shown only compared with WT). (E) Schematic presentation of the FLECS-based single-cell contraction. (F) The ratio of contracted cells was increased in Npas2 KO fibroblasts. (G) Npas2 KO mutation did not affect the gene expression of β-actin (Actb) and α-SMA (Acta2) in dermal fibroblasts. (H) The steady state gene expression level of integrin subunits αV (ItgaV), β3 (Itgb3), and β5 (Itgb5) in dermal fibroblasts was not affected by Npas2 KO mutation.
Fig. 4.
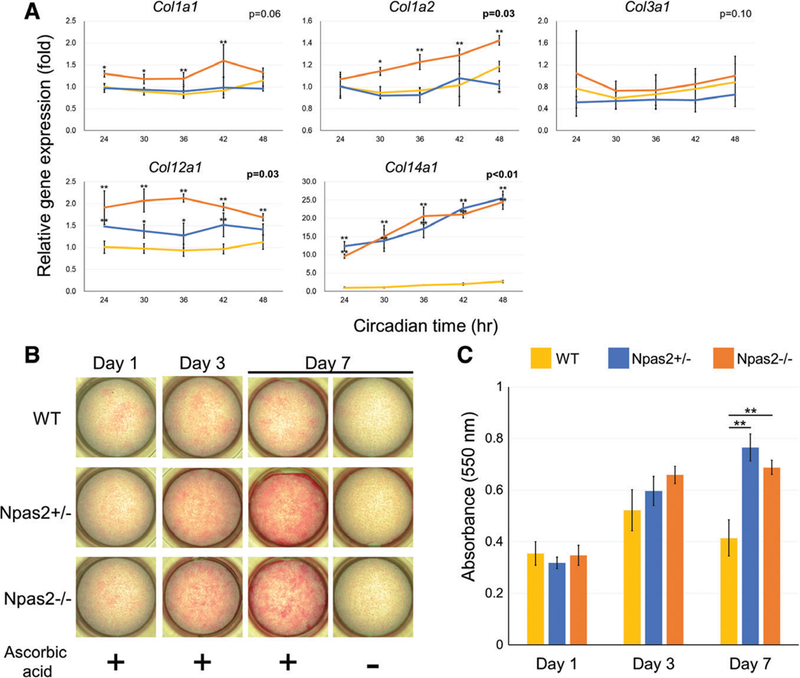
Collagen synthesis by WT, Npas2+/− and Npas2−/− fibroblasts in vitro. (A) Gene expression of collagen type I (Collal and Colla2), type III (Col3a1), type XII (Coll2a1), and type XIV (Coll4a1) (**P < 0.01, *P < 0.05, significant difference shown only compared with WT). FACIT collagen type XII and type XIV showed significantly increased steady-state mRNA levels in Npas2+/− and Npas2−/− fibroblasts. (B) Images for cultured fibroblasts with picrosirius red staining highlighted the synthesis of collagen fibers. (C) The in vitro collagen fiber deposition was measured by picrosirius red staining (**P < 0.01 by one-way ANOVA with post hoc Holm test).
Collagen Synthesis Assessment in vitro by Picrosirius Red Staining
Fibroblasts were seeded into 24-well plates and cultured at 80%−90% confluency in medium supplemented with ascorbic acid (50 μg/mL) for 1, 3, and 7 days. The cells were then fixed with 10% neutral buffered formalin and stained with picrosirius red (PolyScience, Niles, IL) for visualizing the collagen. The absorption value was read in a spectrophotometer at 550 nm with a plate reader (SYHNERGY H1 plate reader) and compared by one-way ANOVA with the post hoc Holm test.
Collagen Fiber Structure at Skin Wound Healing Area by Picrosirius Red Staining and Confocal Laser Scanning Microscopy
The histological sections for the dorsal skin full-thickness wound experiment at 7 and 14 days after surgery were stained with picrosirius red for collagen fibers during wound healing. Collagen fiber structure in the granulation tissue (GT) area, wound closure area (WCA), and intact skin area (ISA) was evaluated using confocal laser scanning microscopy. The distance between the edge of the panniculus car- nosus as the original wound width (a) and the distance between the edge of the maturated collagen at the skin punch area, measured as the width of GT (b), were assessed. The ratio of wound closure was calculated by (a - b)/a and was compared by the Kruskal-Wallis test with Dunn’s post hoc test.
RESULTS
Full-Thickness Dorsal Skin Wound Closure Was Accelerated in Npas2–/– Mice
The full-thickness dorsal skin wounds contracted continuously from Days 2 to 12 after surgery, and scar formation was recognized by Day 12 in all genotypes (Fig. 1A). The relative wound area at Day 12 in Npas2−/− mice was significantly smaller than in the other two genotypes (P < 0.01) (Fig. 1B). In the histological observation, hyperkeratosis, residual clots, and the immune response in the GT area were observed at 7 days after surgery. Furthermore, the edge of dermis connective tissue with hair follicles moved toward the center of wounds by Day 14. The epithelial layer at the wound area appeared to be similar to the intact skin epithelium, and the immune response had declined in all samples (Fig. 1C). In the present study, mouse dorsal skin full-thickness excisional wounds were generated in the middle of the day (11 a.m.−1 p.m.). It has been reported that skin burn wounds occurring during the night or resting period of humans showed impaired healing (Hoyle et al., 2017). Daytime for the nocturnal mice is equivalent to night for humans. It may be possible that the difference in wound healing between WT and Npas2 KO mice might be more evident if the wound occurred during the dark/active period in mice.
Fig. 1.
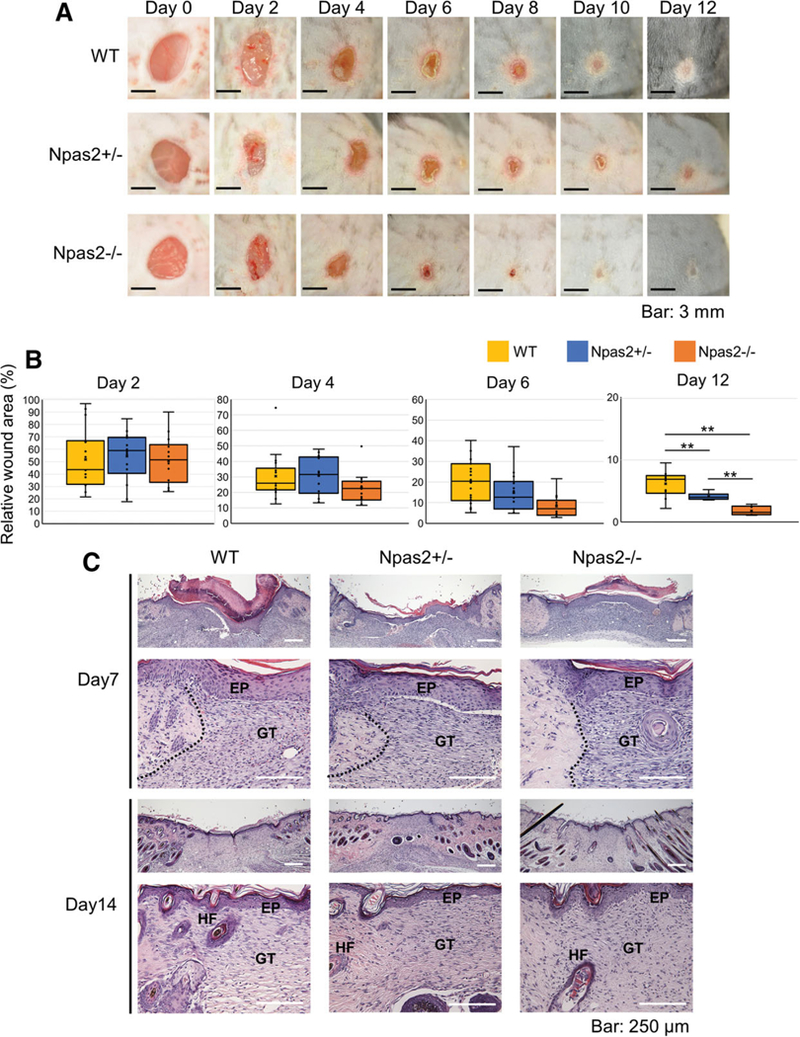
Full-thickness skin punch wound healing. (A) A standardized photograph of the skin wound was obtained from 0 to 12 days after surgery, depicting the progressive wound closure and contraction. (B) The relative wound area was calculated at 2, 4, 6, and 12 days. Npas2 KO mice showed a significantly smaller wound area than that of WT mice at day 12 (**P < 0.01). (C) Histological observation of wounds at Day 7 showed the formation of granulation tissue (GT) and the restoration of epithelial integrity (EP); however, the wound margin (dotted line) was clearly observed. At Day 14, the wound margin highlighted by hair follicles (HFs) was less clear and approached toward the granulation tissue (GT).
The Effect of Npas2 KO Mutation on Proliferation and Circadian Rhythm Gene Expression of Skin Fibroblasts
The genotype for each fibroblast sample was determined by PCR. Exon 2 of the mouse Npas2 allele was replaced by the LacZ expression reporter cassette (LacZ/Neo). Because exon 2 encodes the bHLH sequence, the resultant Npas2 molecule lacked the DNA binding function. The amplified PCR product, which was larger than that of WT, recognized Npas2−/− fibroblasts and both the mutant and WT PCR products recognized Npas2+/− fibroblasts.
The WST-1 assay indicated that both Npas2+/− and Npas2−/− fibroblasts proliferated faster than WT fibroblasts (P < 0.01) (Fig. 2b).
Fig. 2.
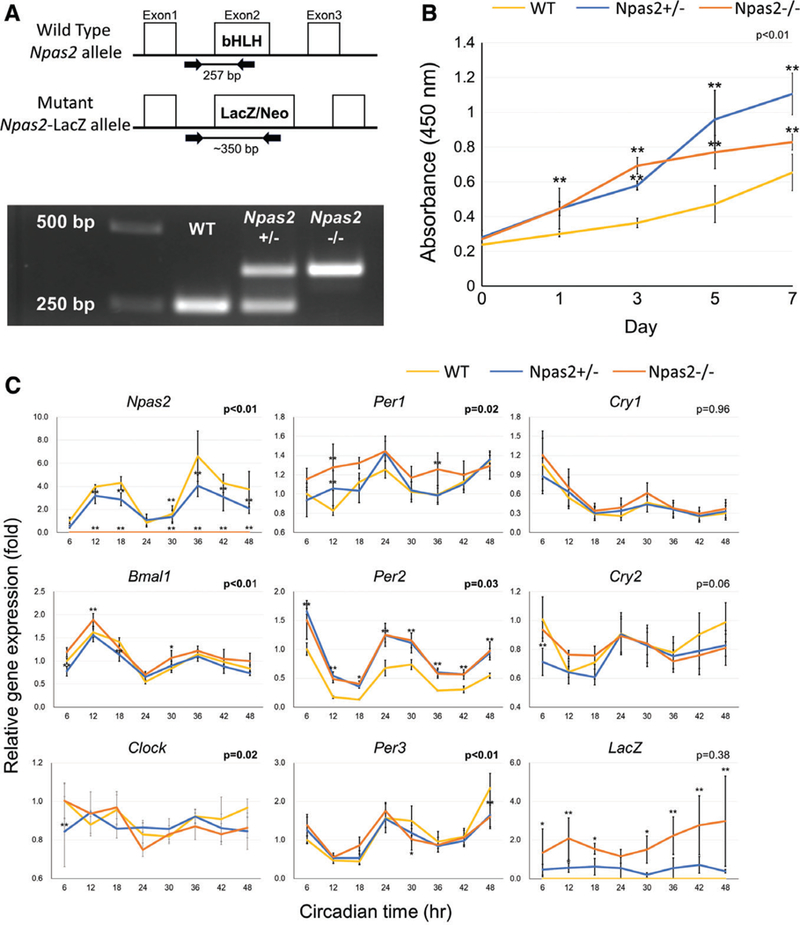
Characterization of WT, Npas2+/−, and Npas2−/− skin fibroblasts. (A) The genotype of each fibroblast batch was determined by genomic DNA PCR. The WT Npas2 allele generated a 250 bp PCR product, whereas the mutant allele generated a 350 bp PCR product. (B) The WST-1 assay demonstrated the increased cell proliferation rate in Npas2 KO fibroblasts (**P < 0.01, significant difference compared with WT at the time points via the Tukey analysis). (C) The expression of core clock genes and the LacZ reporter gene was determined by RT-PCR every 6 for 48 hr (P value in the figure: two-way ANOVA for the interaction between the time and genotype factors. *P < 0.05, **P < 0.01, significant difference compared with WT at the time points via the Tukey analysis) (C).
The circadian expression of Npas2 was decreased in Npas2+/− fibroblasts and was undetected in Npas2−/− fibroblasts. However, an effect of the Npas2 KO mutation on the expression patterns of other circadian genes was not observed, except for the Per2 expression (Fig. 2C). The reporter gene (LacZ expression) was detected only in Npas2 KO mice.
Accelerated in vitro Wound Healing of Npas2–/– Skin Fibroblasts by Scratch Test and Floating Collagen Gel Contraction Assay
The wound healing scratch assay, floating collagen gel contraction assay, and single-cell force assessment with FLECS were performed in vitro. The numbers of migrated Npas2+/− and Npas2−/− fibroblasts were higher than those of WT during 24 hr (Video: https://players.brightcove.net/656326989001/default_default/index.html?videoId=6013197672001), which was statistically significant (P < 0.05) (Fig. 3A,B). However, there was no significant difference between the cell migration rate of Npas2+/− and Npas2−/− fibroblasts (Fig. 3A,B). The floating collagen gel contraction assay showed that Npas2−/− fibroblasts contracted faster than WT and Npas2+/− fibroblasts (P < 0.01) (Fig. 3C,D).
Single-Cell Contraction and Expression of α-SMA and Integrins
The evaluation for single-cell contraction using FLECS (Fig. 3E) revealed that the ratio of contracted Npas2+/− and Npas2−/− fibroblasts was higher than the ratio of WT fibroblasts (P < 0.01) (Fig. 3F). The gene expression levels of β-actin (Actb), known to be related with cell migration, and α-SMA (Acta2), known as the factor for upregulating myofibroblast contractile activity, were evaluated by RT-PCR (Fig. 3G). The expression of both actin subunits decreased over time. However, there was no significant difference among the three genotypes. The expression of integrin αV (ItgaV), integrin β3 (Itgb3), and integrin β5 (Itgb5) did not show any circadian rhythm in dermal fibroblasts. Npas2 KO mutation did not affect the steady-state level of the examined integrin subunits (Fig. 3H).
Npas2–/– Fibroblasts Increased Dermis-Like Collagen Synthesis In Vitro
The gene expression levels of collagen subunits type I (Col1a1, Col1a2), type III (Col3a1), type XII (Col12a1), and type XIV (Col14a1) were investigated in this experiment (Fig. 4A). Overall, no circadian pattern was observed in these collagen mRNAs. Col1a1 and Col1a2 in Npas2−/− fibroblast were more highly expressed than in WT and Npas2+/− fibroblasts; however, the interaction P value was significant only for Col1a2. No difference was observed for the Col3a1 expression. There was an increase of Col12a1 expression in Npas2+/− and Npas2−/− fibroblasts. Strikingly, a significantly elevated expression of Col14a1 was found in both Npas2+/− and Npas2−/− fibroblasts compared to WT fibroblasts. The picrosirius red staining for fibroblasts cultured with ascorbic acid supplementation showed a strong, positive reaction, indicating collagen fiber formation and accumulation in Npas2+/− and Npas2−/− fibroblasts (Fig. 4B); their absorbance at 550 nm was significantly higher than that in WT fibroblasts at Day 7 (P < 0.01) (Fig. 4C).
Dermis-Like Collagen Fiber Reconstruction during Skin Wound Healing of Npas2–/– Mice
Histological sections of the full-thickness skin wound area with picrosirius red staining were examined by con-focal laser scanning microscopy (Fig. 5A). There was no obvious difference in collagen fiber structures in the ISA; however, collagen fibers in both the GT area and the WCA appeared to be thicker in Npas2+/− and Npas2−/− samples than in those of WT. In particular, collagen fibers of GT in Npas2−/− samples appeared more organized, partially resembling the intact skin collagen structure. The histological measurement of wound closure was performed with picrosirius red-stained sections (Fig. 5B). The ratio of wound closure of Npas2+/− and Npas2−/− samples was greater than that of WT, although statistical significance was achieved only between WT and Npas2 −/− samples at Day 14 (P < 0.01) (Fig. 5C).
Fig. 5.
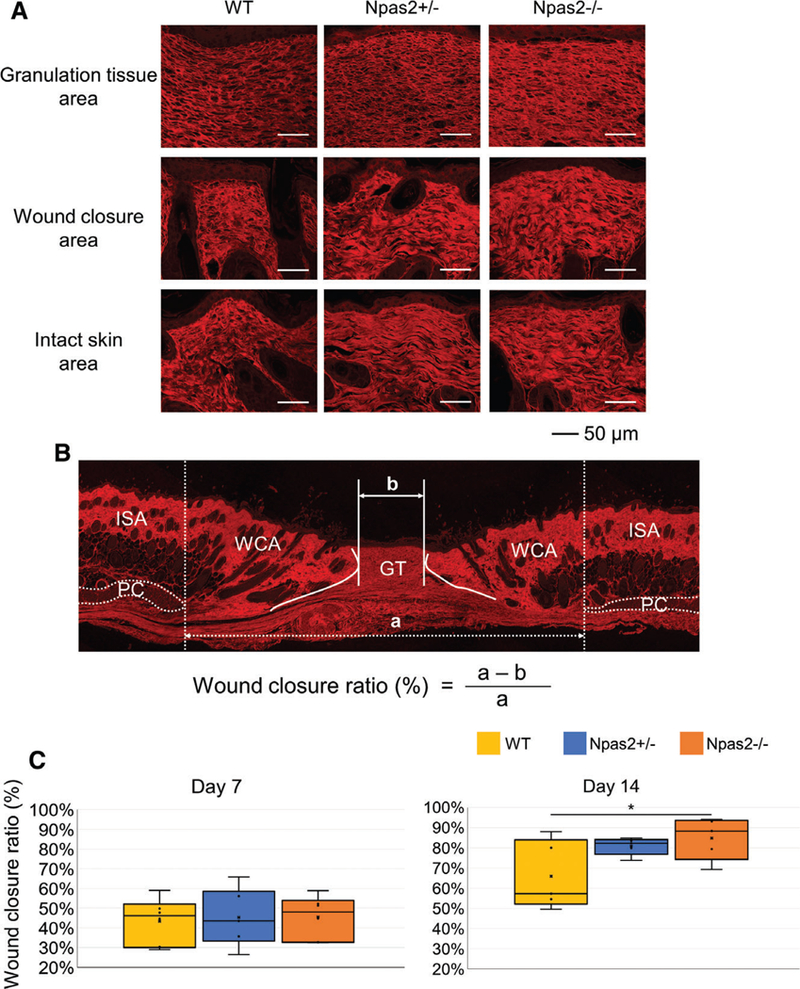
Evaluation of collagen fiber structure in the wound healing area. (A) Confocal laser scanning microscopy depicted the collagen fiber architecture stained with picrosirius red at 14 days after surgery. (B) Measurement of the wound closure ratio using the wound closure area (WCA) calculated as the width of the ISA (a) between panniculus carnosus (PC) subtracted by the granulation tissue area (GT: b), which was normalized by ISA. (C) The wound closure ratio was greater in Npas2+/− and Npas2−/− mice at Day 14, albeit statistical significance was achieved only between the WT and Npas2−/− groups.
DISCUSSION
Mammalian skin is a large barrier tissue composed of the epithelial layer (epidermis) and underlining connective tissue (dermis). This study proposes a novel role of the circadian clock in dermal fibroblasts for skin wound healing, which may possibly enable dermal connective tissue collagen reconstruction. Once injured, the skin epithelial cells actively proliferate and migrate over the wound, leading to the rapid establishment of a barrier layer. By contrast, dermal fibroblasts are slow in proliferation and migration into the wound area. Furthermore, wound fibroblasts do not maintain the dermal fibroblast phenotype, but acquire a new phenotype, in part, contributing to the formation of GT and scarring. The present study demonstrated the accelerated healing of the well- established skin full-thickness wound model (Kowalska et al., 2013) in Npas2+/− and Npas2−/− mice, potentially through faster wound closure and/or smaller scarring than that of WT mice (Fig. 1). As such, this study focused on the role of Npas2 KO mutation on the behavior of dermal fibroblasts as a mechanistic investigation.
Npas2 is a core circadian rhythm gene encoding a basic HLH transcription factor and is highly expressed in skin fibroblasts. Npas2 has been postulated to compensate the role of Clock, whose expression rate in fibroblasts was comparatively low (Fig. 2C) (Landgraf et al., 2016). In the case of retinal cells, knock down of the Clock gene reduced mRNA and protein levels of Npas2, whereas knock down of Npas2 did not affect either the mRNA or protein levels of Clock (Haque et al., 2010). Our data corroborated the previous observation that Npas2 KO mutation did not significantly affect the expression of the core circadian rhythm genes (Fig. 2C). Thus, the effect of Npas2 KO mutation may be mediated by mechanisms other than the disruption of the circadian rhythm. The expression of Npas2 in the SCN peaks at the dark/active period in mice (Haque et al., 2010). Wound responses in mice would be expected to show a daily rhythm. However, we did not explore this issue in the present study.
Three-dimensional collagen gels containing fibroblasts have been used to model tissue remodeling, wound contraction, and fibrosis (Grinnell, 1994). The primary mechanism of fibroblast-embedded gel contraction in vitro is due to fibroblast locomotion forces (Ehrlich and Rajaratnam, 1990). The cell traction force is applied to the substrate ECM, contributing to the collagen gel contraction (Brown et al., 2002). The accelerated collagen gel contraction was demonstrated by Npas2+/− and Npas2−/− fibroblasts (Fig. 3C,D), suggesting the increased fibroblast locomotion forces. It was reported that silencing the NPAS2 expression in human colorectal cancer cells accelerated cell migration (Xue et al., 2014). The present study also showed accelerated migration by Npas2+/− and Npas2−/− fibroblasts in an in vitro scratch test (Video: https://players.brightcove.net/656326989001/default_default/index.html?videoId=6013197672001; Fig. 3A,B). The activation of extracellular signal-regulated kinase (ERK) and phosphoinositide-3 kinase/protein kinase B (PI3K/AKT) through phosphorylation is well known to regulate cell migration, collagen gel contraction, and skin wound healing (Liu et al., 2008; Chen et al., 2014; Li et al., 2016). The activation of these signaling pathways was suggested in the phenotype conversion of fibroblasts toward myofibroblasts, such as an increased expression of α-SMA (Mulero-Navarro et al., 2005). In our study, the phenotype conversion of fibroblasts was not suggested by the Npas2 KO mutation (Fig. 3G), and thus, the involvement of a myofibroblast-like phenotype in the modulated collagen gel contraction and fibroblast migration was ruled out. However, it is important to characterize the effect of Npas2 KO mutation on phosphorylation in the ERK/Akt/FAK pathway.
During migration, fibroblasts adhere to the extracellular matrix (ECM) through integrin molecules and generate a single-cell traction force (Style et al., 2014). We used a recently developed single-cell contraction assay that required cell adhesion to the FLECS printed with fibronectin (Koziol-White et al., 2016; Pushkarsky et al., 2018). The FLECS assay showed that mouse dermal fibroblasts increased the cell contraction behavior by Npas2 KO mutation (Fig. 3F). Wound-induced transformation of fibroblasts to myofibroblasts has been postulated to play a pathological role in tissue contraction and fibrosis formation (Kendall and Feghali-Bostwick, 2014). Separately, the increased expression of alpha and beta integrins mediating cell adhesion to fibronectin were thought to be critical for cell contractility-driven wound fibrosis formation (Conroy et al., 2016). For example, the significantly elevated expression of integrin αVβ3 has been postulated to cause idiopathic pulmonary fibrosis (Fiore et al., 2018). In the present study, the steady-state expression of myofibroblast marker α-SMA as well as integrin subunits αV, β3, and β5 was not altered by Npas2 KO mutation in dermal fibroblasts (Fig. 3G,H). Therefore, the increased fibroblast contractility by Npas2 KO mutation may not result in the abnormal wound healing phenotypes of pathological wound contraction or fibrosis formation.
Connective tissue ECM molecules, in particular the FACIT class of collagens, have been shown to influence cell migration and cell contraction through integrin-mediated cell adhesion (Schiro et al., 1991; Nishiyama et al., 1994; Grässel and Bauer, 2013). The FACIT class of collagens has been postulated to decorate the surface of collagen fibers (Klein et al., 1998). The externally exposed N-terminal globular domains, such as NC3 of type XII and type XIV colla-gens, have been shown to be essential in fibroblast-mediated collagen gel contraction (Nishiyama et al, 1994). Thus, we postulate that the increased expression of type XII and XIV collagens in Npas2 KO fibroblasts might affect the migration and gel contraction behaviors.
It has been reported that downregulation of Npas2 expression is related to cell cycle progression and DNA repair capacity (Zhu et al., 2007; Zhu et al., 2008), although there are conflicting reports on the effect of Npas2 modulation on cell proliferation (Xue et al., 2014; Yuan et al., 2017). Our study indicated that Npas2 KO mutation increased fibroblast proliferation (Fig. 2B), which may have confounded the cell migration assay. When the time-lapse microscopy was evaluated (Video: https://players.brightcove.net/656326989001/default_default/index.html?videoId=6013197672001), no proliferating cells were observed within the scratch area for all genotypes, suggesting that the effect of Npas2 on cell proliferation and migration occurs through mechanisms other than cell proliferation.
Previously, we reported that titanium-based biomaterials increased the Npas2 expression of bone marrow mesenchymal stromal cells (BMSC) concomitantly with an elevated expression of cartilage collagens types II, IX, and X, suggesting that Npas2 might mediate biomaterial-induced BMSC differentiation (Mengatto et al., 2011). Thus, our first step of mechanistic dissection was to examine skin fibroblast differentiation through dermal-related collagens. Skin dermal collagen ECM is primarily composed of fibril-forming type I and type III collagens, which form thick collagen fibers (Gordon and Hahn, 2010). FACIT collagen types XII and XIV have been found in developing skin (Castagnola et al., 1992; Oh et al., 1993; Berthod et al., 1997). Type XII and XIV colla-gens are postulated to decorate the surface of collagen fibers and regulate the physiological ECM organization with tissue-specific functions. By contrast, wound fibroblasts abundantly synthesize collagen ECM with different properties in the GT. Our study revealed a striking upregulation of FACIT collagen types XII and XIV by Npas2 KO fibroblasts (Fig. 4A). The in vitro collagen fiber formation depicted by picrosirius red staining showed thick collagen fibers in the cultures of Npas2+/− and Npas2−/− fibroblasts (Fig. 4B,C). The robustly increased FACIT expression might contribute to the re-organization of dermis-like collagen fibers in the skin wound. In fact, Npas2−/− mice demonstrated an increased WCA containing mature dermis-like collagen structure (Fig. 5A). Furthermore, the GT of Npas2−/− mice showed thicker collagen fibers, in part, resembling the dermis-like collagen fiber structure. Taken together, we propose that fibroblasts with decreased Npas2 expression may differentiate to dermal fibroblasts, not myofibroblasts or GT fibroblasts, and Npas2-suppressed fibroblasts might induce their ability to better reconstruct, if not partially regenerate, the dermal collagen architecture.
CONCLUSION
Our study demonstrated that Npas2 suppression in peripheral skin fibroblasts modified cell behaviors and was depicted by accelerated cell proliferation, cell migration, and cell contraction forces in vitro. Moreover, Npas2 suppression resulted in increased dermis FACIT collagen synthesis and the formation of thick collagen fibers. These fibroblastic phenotypes appeared to have contributed to better skin wound healing and the potential reconstruction of dermis collagen architecture. Within the scope of this article, the mechanism of circadian clock molecules, such as Npas2, in dermal wound healing may facilitate skin-specific cell differentiation. From these results, we propose that Npas2 may be an attractive therapeutic target for improving skin wound healing.
Supplementary Material
ACKNOWLEDeGEMENTS
We thank Dr. Christopher S. Colwell and Dr. Yu Tahara, Department of Psychiatry & Biobehavioral Science, David Geffen, School of Medicine at UCLA, Los Angeles, CA for data interpretation of clock gene expression. We also thank Dr. Ivan Pushkarsky, Department of Bioengineering, UCLA Henry Samueli School of Engineering and Applied Science, Los Angeles, CA for technical assistance of the single-cell contraction assay. This study was supported, in part, by a UCLA Faculty Seed Grant (IN) and conducted in a facility constructed with support from the Research Facilities Improvement Program Grant Number C06 RR014529 from the National Center for Research Resources, National Institutes of Health (NCRR/NIH).
Grant sponsor: NIH/NCRR; Grant number: C06 RR014529; Grant sponsor: UCLA School of Dentistry; Grant number: Faculty Seed Grant.
Footnotes
This article includes AR WOW Video. Video can be viewed at https://players.brightcove.net/656326989001/default_default/index.html?videoId=6013197672001.
LITERATURE CITED
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. 2002. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12: 540–550. [DOI] [PubMed] [Google Scholar]
- Berthod F, Germain L, Guignard R, Lethias C, Garrone R, Damour O, van der Rest M, Auger FA. 1997. Differential expression of collagens XII and XIV in human skin and in reconstructed skin. J Invest Dermatol 108:737–742. [DOI] [PubMed] [Google Scholar]
- Bertolucci C, Cavallari N, Colognesi I, Aguzzi J, Chen Z, Caruso P, Foá A, Tosini G, Bernardi F, Pinotti M. 2008. Evidence for an overlapping role of CLOCK and NPAS2 transcription factors in liver circadian oscillators. Mol Cell Biol 28:3070–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Sethi KK, Gwanmesia I, Raemdonck D, Eastwood M, Mudera V. 2002. Enhanced fibroblast contraction of 3D collagen lattices and integrin expression by TGF-beta1 and -beta3: mechanoregulatory growth factors? Exp Cell Res 274:310–322. [DOI] [PubMed] [Google Scholar]
- Bunger M, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. 2000. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnola P, Tavella S, Gerecke DR, Dublet B, Gordon MK, Seyer J, Cancedda R, van der Rest M, Olsen BR. 1992. Tissue-specific expression of type XIV collagen-- a member of the FACIT class of collagens. Eur J Cell Biol 59:340–347. [PubMed] [Google Scholar]
- Chen JC, Lin BB, Hu HW, Lin C, Jin WY, Zhang FB, Zhu YA, Lu CJ, Wei XJ, Chen RJ. 2014. NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways. Biomed Res Int 2014:547187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy KP, Kitto LJ, Henderson NC. 2016. aV integrins: key regulators of tissue fibrosis. Cell Tissue Res 365:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HP, Rajaratnam JB. 1990. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 22:407–417. [DOI] [PubMed] [Google Scholar]
- Fiore VF, Wong SS, Tran C, Tan C, Xu W, Sulchek T, White ES, Hagood JS, Barker TH. 2018. Avb3 integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight 3(20):e97597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni A, Markova-Car E, Dević-Pavlić S, Jurišić D, Puppin C, Mio C, De Luca M, Petruz G, Damante G, Pavelić SK. 2017. A polymorphic GGC repeat in the NPAS2 gene and its association with melanoma. Exp Biol Med 242:1553–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles-Gonzalez MA, Gonzalez G. 2004. Signal transduction by heme-containing PAS-domain proteins. J Appl Physiol 96: 774–783. [DOI] [PubMed] [Google Scholar]
- Glass D, Viñuela A, Davies MN, Ramasamy A, Parts L, Knowles D, Brown AA, Hedman AK, Small KS, Buil A, et al. 2013. Gene expression changes with age in skin, adipose tissue, blood and brain. Genome Biol 14:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Hahn RA. 2010. Collagens. Cell Tissue Res 339:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassel S, Bauer RJ. 2013. Collagen XVI in health and disease. Matrix Biol 32:64–73. [DOI] [PubMed] [Google Scholar]
- Grinnell F 1994. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Ali FG, Biscoglia R, Abey J, Weller J, Klein D, Iuvone PM. 2010. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem 113:1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle NP, Seinkmane E, Putker M, Feeney KA, Krogager TP, Chesham JE, Bray LK, Thomas JM, Dunn K, Blaikley J, et al. 2017. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci Transl Med 9:eaal2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall RT, Feghali-Bostwick CA. 2014. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Kibbler C, Schermutzki F, Brown J, Muller CA, Timpl R. 1998. Cell binding properties of collagen type XIV for human hematopoietic cells. Matrix Biol 16(6):307–317. [DOI] [PubMed] [Google Scholar]
- Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C, Brown SA. 2013. NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci USA 110:1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol-White CJ, Yoo EJ, Cao G, Zhang J, Papanikolaou E, Pushkarsky I, Andrews A, Himes BE, Damoiseaux RD, Liggett SB, et al. 2016. Inhibition of PI3K promotes dilation of human small airways in a rho kinase-dependent manner. Br J Pharmacol 173: 2726–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Wang LL, Diemer T, Welsh DK. 2016. NPAS2 Compensates for Loss of CLOCK in Peripheral Circadian Oscillators. PLoS Genet 12:e1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Li YY, Sun JE, Lin WH, Zhou RX. 2016. ILK-PI3K/AKT pathway participates in cutaneous wound contraction by regulating fibroblast migration and differentiation to myofibroblast. Lab Invest 96:741–751. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ko JA, Yanai R, Kimura K, Chikama T, Sagara T, Nishida T. 2008. Induction by latanoprost of collagen gel contraction mediated by human tenon fibroblasts: role of intracellular signaling molecules. Invest Ophthalmol Vis Sci 49:1429–1436. [DOI] [PubMed] [Google Scholar]
- Mengatto CM, Mussano F, Honda Y, Colwell CS, Nishimura I. 2011. Circadian rhythm and cartilage extracellular matrix genes in ossoeintegration: a genome-wide screening of implant failure by vitamin D deficiency. PLoS One 6(1):e15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. 2001. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105:877–889. [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. 2004. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulero-Navarro S, Pozo-Guisado E, Perez-Mancera PA, Alvarez- Barrientos A, Catalina-Fernandez I, Hernandez-Nieto E, Saenz-Santamaria J, Martinez N, Rojas JM, Sanchez-Garcia I, et al. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J Biol Chem 280(31):28731–28741. [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, NaefF Schibler U., 2004. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119: 693–705. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, McDonough AM, Bruns RR, Burgeson RE. 1994. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J Biol Chem 269(45):28193–28199. [PubMed] [Google Scholar]
- Ngo P, Ramalingam P, Phillips JA, Furuta GT. 2006. Collagen gel contraction assay. Methods Mol Biol 341:103–109. [DOI] [PubMed] [Google Scholar]
- Oh SP, Griffith CM, Hay ED, Olsen BR. 1993. Tissue-specific expression of type XII collagen during mouse embryonic development. Dev Dyn 196(1):37–46. [DOI] [PubMed] [Google Scholar]
- Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. 2006. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett 580:127–130. [DOI] [PubMed] [Google Scholar]
- O’Neil D, Mendez-Figueroa H, Mistretta TA, Su C, Lane RH, Aagaard KM. 2013. Dysregulation of Npas2 leads to altered metabolic pathways in a murine knockout model. Mol Genet Metab 110:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkarsky I, Tseng P, Black D, France B, Warfe L, Koziol-White CJ, WFJr J, Trinh RK, Lin J, Scumpia PO, et al. 2018. Elastomeric sensor surfaces for high-throughput single-cell force cytometry. Nat Biomed Eng 2:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. 1991. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell 67:403–410. [DOI] [PubMed] [Google Scholar]
- Sipahi M, Zengin K, Tanik S, Arslan E, Qubukgu A. 2014. Effects of circadian rhythm disorders on wound healing and strength of bowel anastomosis in rats. Wounds 26:317–322. [PubMed] [Google Scholar]
- Style RW, Boltyanskiy R, German GK, Hyland C, MacMinn CW, Mertz AF, Wilen LA, Xu Y, Dufresne ER. 2014. Traction force microscopy in physics and biology. Soft Matter 10:4047–4455. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. 1994. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 64:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen H, Young MW. 2006. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet 40:409–448. [DOI] [PubMed] [Google Scholar]
- Xue X, Liu F, Han Y, Li P, Yuan B, Wang X, Chen Y, Kuang Y, Zhi Q, Zhao H. 2014. Silencing NPAS2 promotes cell growth and invasion in DLD-1 cells and correlated with poor prognosis of colorectal cancer. Biochem Biophys Res Commun 450:1058–1062. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. 2004. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Li J, Zhou F, Huang Q, Zhang J, Guo X, Lyu Z, Zhang H, Xing J. 2017. NPAS2 promotes cell survival of hepatocellular carcinoma by transactivating CDC25A. Cell Death Dis 8:e2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Barnard M, Tian H, Li X, Ring HZ, Francke U, Shelton J, Richardson J, Russell DW, McKnight SL. 1997. Molecular characterization of two mammalian bHLH-PAS domain proteins selectively expressed in the central nervous system. Proc Natl Acad Sci USA 94:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Leaderer D, Guss C, Brown HN, Zhang Y, Boyle P, Stevens RG, Hoffman A, Qin Q, Han X, et al. 2007. Ala394Thr polymorphism in the clock gene NPAS2: a circadian modifier for the risk of non- Hodgkin’s lymphoma. Int J Cancer 120:432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Stevens RG, Leaderer D, Hoffman A, Holford T, Zhang Y, Brown HN, Zheng T. 2008. Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res Treat 107:421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


