Abstract
Background
Interpersonal stress and perceived rejection have been clinically observed as common triggers of nonsuicidal self-injury (NSSI), with self-injury behavior regulating both affective and social experiences. We investigated whether the subjective interpretation of social interaction in a simulated online environment might be biased in the NSSI group, and the brain mechanisms underlying the experience.
Methods
Thirty female adolescent patients with NSSI and thirty female age-matched controls were investigated in this case–control study. In our novel task that simulates interaction on current social media platforms, participants indicated whether they liked or disliked pictures of other players during a functional magnetic resonance imaging (fMRI) scan. Participants also viewed positive and negative feedback directed toward them by others. The task also assessed the subjective effects of the social interaction. Finally, subjects underwent a separate facial electromyography session, which measured facial expressions processing.
Outcomes
Behaviorally, the NSSI group showed a negative bias in processing social feedback from others. A multi-voxel pattern analysis (MVPA) identified brain regions that robustly classified NSSI subjects and controls. Regions in which mutual activity contributed to the classification included dorsomedial prefrontal cortex and subgenual anterior cingulate cortex, a region implicated in mood control. In the NSSI group, multi-voxel classification scores correlated with behavioral sensitivity to negative feedback from others. Results remained significant after controlling for medication, symptoms of depression, and symptoms of borderline personality disorder.
Interpretation
This study identified behavioral and neural signatures of adolescents with NSSI during social interaction in a simulated social media environment. These findings highlight the importance of understanding social information processing in this clinical population and can potentially advance treatment approaches.
Keywords: NSSI, fMRI, mvpa, Social interaction
Research in context
Evidence before this study
Although interpersonal stress has been clinically observed as a common trigger of nonsuicidal self-injury (NSSI), behavioral and neural mechanisms underlying social processing in adolescents with NSSI remain relatively unexplored in experimental settings. To identify previous studies which addressed behavioral and brain correlates to social processing in individuals with NSSI, we entered the following search items in PubMed: “NSSI”, “fMRI” and “social”. We found three articles, of which two included brain imaging studies. In the two brain imaging studies, which used the same NSSI adolescent sample, participants were selected based on the psychiatric diagnoses of depression with or without borderline personality disorder. In the current study, which provides a larger sample size than the aforementioned studies, we selectively included individuals with NSSI, independent of psychiatric diagnosis.
Added values of this study
In this study, individuals with NSSI experienced evaluative social feedback more negatively than controls following participation in a task which simulated online social interaction. In addition, multivoxel pattern analysis (MVPA) of neural-response data yielded a significant classification of individuals with NSSI and classification indices correlated selectively in individuals with NSSI with elevated sensitivity to negative social feedback. To our knowledge, this is the first study to identify significant behavioral and brain differences in processing social information in individuals with NSSI (independent of psychiatric diagnosis), compared to controls.
Implications of all the available evidence
Using an ecologically valid social interaction task, we provide novel behavioral and neural-functional insights into how individuals with NSSI experience social interactions.
Alt-text: Unlabelled Box
1. Introduction
Nonsuicidal self-injury (NSSI) is defined as the direct, deliberate destruction of one's own body tissue without suicidal intent [1], typically including behaviors such as cutting, burning, or hitting oneself. The risk of engaging in NSSI is particularly high during adolescence, with prevalence rates around 17% in community samples [2] and between 40 and 80% in clinical samples [3]. NSSI is more common in females, especially in clinical samples [4]. The behavior is often related to distress and/or functional impairment, and can occur together with or independently of psychiatric diagnoses, including depression and anxiety [5]. Diagnostically, it is currently a symptom of borderline personality disorder (BPD) [6]. NSSI is far more prevalent than BPD in adolescents [2], [3], suggesting that NSSI can exist independently of or co-occur with BPD. NSSI has been suggested as a diagnostic entity of its own and included in the third section of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [6] as a condition requiring further study, highlighting the importance of research in this area.
Interpersonal stress, perceived criticism, and social rejection are common triggers of NSSI. Social interactions are essential for well-being across the lifespan, but particularly so during adolescence, when socialization into peer-groups increases the importance of social interactions [7]. The age of onset for NSSI is around 12–14 years [3] and peaks during adolescence, with rates declining in adulthood [8]. Sensitivity to interpersonal stress, high emotional distress and in some cases chronic romantic stress [9] have been suggested to play a role in the development and maintenance of NSSI, with self-injury behavior regulating both affective and social experiences [1]. The fact that interpersonal difficulties commonly precede NSSI has also been noted in the proposed DSM-5 criteria [6]. A role of interpersonal stress in triggering NSSI is supported by studies using semi-structured interviews and ecological momentary assessment (EMA). Adolescents with NSSI report greater perceived stress compared to controls when completing an interpersonal conflict task, as well as greater interpersonal sensitivity, such as separation anxiety and fragile inner self [10]. EMA studies have identified an association between NSSI and interpersonal instability [11]. Moreover, Nock et al. have shown that NSSI-related thoughts in adolescents are often triggered while socializing with others and the likelihood of NSSI is greater as a function of increased perceived rejection [12]. Thus, understanding psychological and neural mechanisms through which social stress triggers NSSI is likely to improve the understanding of NSSI.
Clinical observations and correlational data suggest a relationship between social interaction processing and NSSI-thoughts and behavior in adolescence, but no studies have shown these effects under controlled experimental conditions. A small number of prior neuroimaging studies identified brain regions of potential importance for feelings of social rejection in adolescent NSSI, but these studies are preliminary in nature due to their small sample sizes. In addition, their samples were based on psychiatric diagnoses, such as depression or BPD, rather than NSSI per se [13]. These studies did not identify significant behavioral effects. Neural mechanisms underlying NSSI in adolescents remain largely unknown.
Here, we used an experimental approach to investigate the behavioral and neural mechanisms underlying the clinical symptomatology of NSSI. We sought to answer two key questions: First, do adolescents with NSSI perceive social interactions, more negatively than healthy controls? Second, can neural activity elicited during social interactions discriminate NSSI from healthy controls?
To address these questions, adolescents with NSSI and healthy controls engaged in a simulated online social-interaction paradigm in an MRI environment [14].
Recent data show that approximately 90% of Swedish adolescents and young adults (15–24 years) use social media daily, with an estimated 10 h spent weekly on sharing photos and videos with others, giving and receiving evaluations of posted messages by means of “likes,” etc. [15]. Social media platforms are regularly used as a form of interaction, indicating the ecological validity of our approach.
Brain correlates of the social interaction were assessed, as well as subjective perception of the interaction. In order to rule out a potential “negativity bias” in emotional processing, participants also completed a session assessing behavioral and facial-electromyographic responses to dynamically developing emotional faces. Using this approach, we measured behavioral responses to social interaction, and patterns of brain activity that discriminated between the two groups, while controlling for processing of facial expressions.
2. Materials and Methods
2.1. Participants
Thirty healthy controls (mean age, 16·4 years, SD 0·9) were recruited through advertisement in schools and on Facebook. Thirty clinical participants (mean age, 15·9 years, SD 0·8) were recruited from the child and adolescent psychiatric (CAP) clinic at Linköping University Hospital, Sweden. Participants completed two sessions on separate days, in a counterbalanced order. One session included an fMRI scan, and the other assessed behavior and facial electromyography (EMG). Three participants per group were excluded from the MRI session because of stress associated with the session or use of dental braces. Twenty-seven patients and 27 age-matched controls were thus included in the MRI analysis, and 30 patients and controls completed the facial EMG session.
Inclusion criteria for the clinical participants were: NSSI, independent of psychiatric diagnosis, being a female between 15 and 18 years, and having engaged in five or more instances of NSSI during the last six months. Exclusion criteria were: current or life-time diagnosis of schizophrenia, bipolar or psychotic disorder and/or alcohol/drug dependence, and IQ below 80. Healthy controls were included if they had no DSM Axis I or II disorder during the last year and no lifetime presence of NSSI. Patients taking psychotropic medications were included provided that they were ongoing and unchanged for at least three months; sensitivity analyses were subsequently carried out to examine the potential confounding role of medication. Eleven patients were taking psychotropic medications (Table 1). Adolescents meeting inclusion criteria were approached with oral and written information about the study. Participants (and parents, if the participant was < 18) gave written informed consent. Clinical recruitment occurred from June 2016 to March 2018. Controls and clinical participants did not differ in age, IQ or handedness. Additional information is presented in Table 1. The study was approved by the Regional Ethical Board of Linköping (Dnr 2015/273-31; 2016/224-32). To ensure that any adverse reactions, such as worry or anxiety, were managed according to clinical routine, all participants were accompanied to the sessions by the same clinician that had provided their initial diagnosis.
Table 1.
Participant demographics.
| Demographic characteristics | NSSI n = 26–30 n (%) |
Healthy controls n = 26–30 n (%) |
Comparison statistic |
|---|---|---|---|
| Sex | |||
| Female | 30 (100%) | 30 (100%) | |
| Age | |||
| m (sd) | 15.9 (0.79) | 16.4 (1.0) | n.s. |
| IQ | |||
| m (sd) | 96.6 (9.83) | 100.9 (10.94) | n.s. |
| Handedness | |||
| m (sd) | 77.9 (29.88) | 78.5 (43.20) | n.s. |
| Parental education (NSSI n = 55, control n = 53) |
|||
| University/college | 23 (41.8%) | 32 (60.4%) | n.s. |
| Theoretical high-school program | 5 (9.1%) | 6 (11.3%) | |
| Vocational high-school program | 23 (41.8%) | 13 (24.5%) | |
| Compulsory school | 4 (7.3%) | 2 (3.8%) | |
| Parent born in other country (NSSI n = 57, control n = 56) |
4 (13.8%) | 5 (17.9%) | n.s. |
| Current family structure | |||
| Married/co-habitant | 12 (40.0%) | 18 (62.1%) | n.s. |
| Divorced | 18 (60.0%) | 10 (34.5%) | |
| Single parent household | 0 (0%) | 1 (3.4%) | |
| Depressive symptoms (CDRS-R) m (sd) | 45.7 (13.36) | 22.2 (4.95) | p < 0.001 Cohens d = 2.33 |
| NSSI | |||
| DSM-5 NSSID diagnosis | 18 (62.1%) | ||
| Age of onset m (sd) | 13.2 (1.25) | ||
| Number of methods m (sd) | 3.8 (2.13) | ||
| Cutting frequency (12 months) m (sd) | 54.6 (55.7) | ||
| Latest NSSI episode (weeks) m (sd) | 3.5(5.15) | ||
| Suicidal behaviors | |||
| Suicide ideation | 30 (100%) | ||
| Suicide attempt | 11 (36.7%) | ||
| Ever inpatient psychiatric care | 7 (23.3%) | ||
| SCID-II self-report m (sd) | 6.00 (2.8) | 1.07 (1.5) | p < 0.001 Cohens d = 2.19 |
| SCID-II interview m (sd) | 3.23 (2.6) | 0.13 (0.51) | p < 0.001 Cohens d = 1.65 |
| Psychiatric diagnoses* | |||
| Depression | 15 (50.0%) | ||
| Anxiety disorder | 13 (43.3%) | ||
| Posttraumatic stress disorder | 1 (3.3%) | ||
| Borderline traits | 13 (43.3%) | ||
| Eating disorder | 6 (20.0%) | ||
| ADHD/ADD | 15 (50.0%) | ||
| High functioning autism | 4 (13.3%) | ||
| ODD/CD | 3 (10.0%) | ||
| Medications** | |||
| SSRI/SNRI | 8 (26.7%) | ||
| SSRI/SNRI + methylphenidate | 1 (3.3%) | ||
| Neuroleptic | 1 (3.3%) | ||
| SSRI/SNRI + neuroleptic | 1 (3.3%) | ||
| No medication | 19 (63.3%) |
Note. *each participant could have several diagnoses **medication at time of fMRI.
2.2. Psychometric Measures
We used the clinical interview Schedule for Affective Disorders and Schizophrenia for School-Age-Children-Present and Lifetime (K-SADS-PL) [16] for DSM-IV diagnoses; selected questions from the semi-structured Self-injurious thoughts and behaviors interview (SITBI [17], [18]) to obtain detailed information about suicidal behavior; the Structural Clinical Interview for DSM-IV Personality Disorders (SCID-II) [19] for symptoms of borderline personality disorder; Children's Depressive Rating Scale – Revised (CDRS-R) [20] for depressive symptoms; an abbreviated version of Wechsler Intelligence Scales, fourth edition for children [21] or adults [22], depending on participants' age, for intelligence. For the clinical sample only Clinical Assessment of Nonsuicidal Self-Injury Disorder (CANDI) [23] assessed NSSI characteristics (including frequency and means of NSSI) and whether NSSI participants met criteria for a diagnosis of NSSI disorder. Assessments were performed by the last author, a clinical psychologist with extensive experience in psychiatric assessment, together with the second author, a child psychiatrist. Final psychiatric diagnoses for the clinical sample were based on all available information from diagnostic interviews and medical records, using DSM-5 [6].
2.3. fMRI Task
In the scanner, subjects participated in a simulated online game aimed at identifying neural regions involved in the processing of self-relevant information during social interaction [14]. Briefly, participants were presented with pictures of other adolescents, and asked to indicate whether they liked or disliked the person. Similarly, participants viewed their own picture being judged by other simulated players. The event-related design consisted of trials comprising three epochs: question, anticipation and outcome. For full details on the design see Perini et al. [14].
Questionnaires were completed immediately following the MRI scan to assess subjective perception of the social interaction and its effects on participants. Question one assessed the perceived ratio of negative feedback (“How often were you disliked?” 0% “never”-100% “always”) when, in fact, the task was balanced so that the participants received equal amounts of positive and negative feedback. Question two asked: “How much did you like to see your own face?” (0 “not at all”-10 “very much”). Finally, we investigated the emotional effects of being liked or disliked by others with the following questions: “How bad did it feel to be disliked?” (0 “not at all” – 10 “very much”) and “How good did it feel to be liked?” (0 “not at all” – 10 “very much”). Scores' residuals were tested for normality. Depending on whether the assumption of normality was met or not, individual scores for each group were compared using respectively a univariate ANOVA or a Mann–Whitney test, respectively. Either Pearson's or Spearman's correlation coefficients were calculated depending on whether data met assumptions of normality. Behavioral statistics were calculated using the Statistical Package for the Social Sciences (SPSS) version 25.
2.4. Image Acquisition
Imaging was performed using a Philips Ingenia 3 Tesla MR scanner (Philips Healthcare, Best, The Netherlands) equipped with a 32-channel Philips dS Head head-coil. Six dummy volumes were acquired before each scan to allow the spin system to reach steady-state longitudinal magnetization and reduce possible effects of partial saturation. Blood oxygen-level-dependent (BOLD) data were acquired with an echo-planar imaging (EPI) sequence: TR = 2000 ms; TE = 30 ms; flip angle = 77°; field-of-view = 220 × 220 mm2; in-plane resolution = 3.4 × 3.4 mm; slice thickness = 4 mm, no slice gap; number of axial slices (angled with the AC-PC line) = 32; number of volumes = 195. Two functional runs were collected and each run lasted for 6 min and 45 s. A high-resolution 3D T1-weighted Turbo Field Echo scan was acquired before the EPI data acquisitions: TR = 7.0 ms; TE = 3.2 ms; flip angle = 8°; field-of-view = 256 × 240 × 170 mm; voxel resolution = 1 × 1 × 1 mm; no slice gap; plane: sagittal; number of sagittal slices = 170.
2.5. Image Preprocessing
Preprocessing was performed with the Analysis of Functional Neuro Images (AFNI) software v16.2.12 [24]. BOLD images were de-spiked and slice-time corrected. For motion correction and co-registration purposes, each EPI volume was registered to the volume with the minimum outlier fraction (using the AFNI outlier definition). Functional images were then warped to Talairach template space using a combination of affine and non-linear transformations [25]. Nuisance effects due to head motion (estimated from the motion correction procedure) were accounted for by adding the motion parameters (and their derivatives) as regressors of no interest in the main regression. A motion censoring threshold of 0.3 mm per TR was implemented in combination with an outlier fraction threshold of 0.1. Volumes violating either of these thresholds were subsequently ignored in the time-series regression. There was no statistical difference in the amount of volumes ignored in the two groups (t = − 1.1, p = 0.2, two-tailed). For the participants with volume censoring over 30% of the total volume number, we found no significant difference in the censoring per condition for either anticipation (p = 0.1) and outcome (p = 0.7) intervals. Table S1 shows percentage of removed volumes for each participant.
2.6. Univariate Analysis
A univariate general linear model (GLM) analysis described elsewhere [14] was initially performed to identify brain correlates of self-relevant processing and to assess replication of previous findings [14]. The AFNI program 3dClustSim was used to determine cluster-size thresholds necessary for identifying effects significant at alpha = 0·05, family-wise-error corrected (per voxel p < 0·002, two-sided; cluster threshold = 30 voxels), according to current recommendations by AFNI developers [26], [27]. Average spatial smoothness estimates, across all participants, and entered into 3dClustSim were obtained using the 3dFWHMx function with the ACF option.
2.7. Multi-voxel Pattern Analysis
We applied a multivariate machine-learning approach, first, to determine whether brain activity during the online-game task could be used to classify group membership and, second, to identify brain regions that most significantly contributed to multivariate discrimination of groups. We applied a support vector machine (SVM) to voxel-wise indices of task-activation using in-house software developed in MATLAB 10.6 (www.mathworks.com) calling both MATLAB and AFNI functions [24].
The machine-learning approach was applied, voxel-wise, to neural-response indices derived from traditional GLM “task-based” analysis approaches that were applied to BOLD time-series data from both anticipation and outcome epochs. We used leave-one-out cross validation to determine the diagnostic accuracy of the approach. With this method, all participants but one is used to train the classifier, and the remaining subject's data is used to test the classifier. We used a simple, linear-kernel SVM with margin softness parameter, c, set to 1. We used random permutation testing (1000 permutations) to determine the significance of classifier accuracy achieved. To depict classifier performance, a receiver operating characteristic (ROC) curve was generated in SPSS using classification scores. To identify brain regions that reliably contributed to discrimination between healthy and NSSI, we performed the following steps: The resulting brain maps from the permutation testing were transformed into z-scores by subtracting the average permutation weight scores from the classification weight scores and dividing the resulting difference by the standard deviation of the permutation weight scores. We used AFNI's 3dClustSim (spatial smoothness estimated with 3dFWHMx, ACF option) to determine cluster-size thresholds necessary for identifying effects significant at per-voxel p = 0·002 (two-sided; z = 2·89), family-wise-error corrected at alpha = 0·05 (cluster size = 6). We conducted this MVPA approach twice, once on voxels from a whole-brain, grey-matter mask and, again, using this same mask but without regions activated for the self-versus-other contrast during anticipation (i.e. anterior insula, dorsal anterior cingulate cortex, and supplementary motor area). We did this to determine if brain regions that are “silent” in the univariate, task-activation sense might, at a multivariate level, still effectively discriminate disordered from healthy samples.
Finally, to evaluate the clinical relevance of our machine-learning approach, we examined in the NSSI group the association between sensitivity to rejection as measured by post-game self-report and the classification score generated for each member of the NSSI group (i.e. Euclidian norm distance between individual-subject data and the trained support vector) when their data were classified during leave-one-out cross-validation. We used partial correlations to estimate the association between the MVPA classification score and rejection sensitivity controlling for BPD traits, assessed using the SCID II interview, depression scores, assessed using the CDRS-R scale, and medication use.
2.8. Facial Electromyography
Emotions sequences included happiness, sadness, surprise, fear, anger, and disgust comprising 15 static morphed images displayed for 200 ms each that conveyed from 5% emotion to 100% emotion. Stimuli were created as previously described [28]. Briefly, young men and women (n = 12 each gender) posed each emotion and were photographed; composite images of each of the six emotions were created for each gender [29], [30]. Each gender-by-emotion sequence was displayed four times for a total of 48 trials presented in pseudorandom order. Participants were instructed to press a button as soon as they could identify the emotion (“sensitivity”) and then given a list of all six emotions to choose from to identify the emotion (“accuracy”). Facial EMG recordings of the corrugator and zygomatic muscles were obtained simultaneously.
Recordings were obtained as previously described [31]. Briefly, sensors consisted of bipolar 4 mm silver/silver chloride electrodes filled with electrode gel placed on the left side of the face and a ground electrode on the forehead. EMG signals were amplified, filtered through a 10–500 Hz band pass, digitized at 1 kHz, rectified, and integrated over 20 ms using the MP150 Data Acquisition system, and Acqknowledge software from Biopac Systems (Biopac Systems Incorporated, Camino Goleta, CA, USA). To control for the varied duration of trial length (e.g. how long it took the participant to identify the emotion), we analyzed the mean EMG activity during the final 1000 ms of the stimulus display compared to the mean activity during the 1000-millisecond baseline immediately prior to trial onset [32].
Behavioral outcomes (sensitivity, accuracy) and mean facial EMG activity (corrugator, zygomatic) were analyzed using a repeated-measures analysis of variance (ANOVA) with the within-subject factor of emotion and the between-subject factor of group. A secondary analysis assessed reactions to each emotion individually, accounting for the emotional intensity at the time of trial termination [29].
2.9. Data Statement
We do not have participants' consent to share raw data publicly. The data that has been used is confidential.
3. Results
3.1. Behavioral Findings
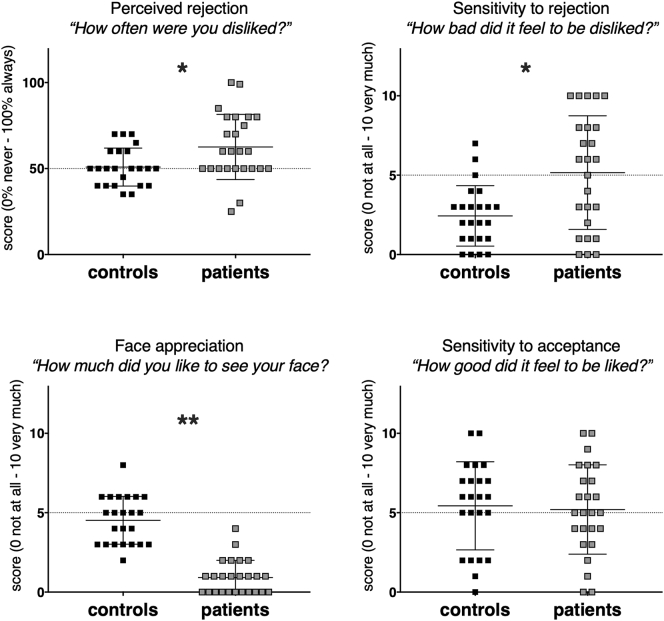
Based on post-scan queries, NSSI patients felt rejected significantly more often than controls [(U = 172, p = 0·009, r = 0·37); Fig. 1] and, at trend level, also disliked others, more often than controls (F(0,52) = 11·5, p = 0·08, η2p = 0·05). Compared to controls, NSSI individuals were also significantly more sensitive to being rejected and disliked significantly more to see their own face during the game (sensitivity to rejection F(0,47) = 11·5, p = 0·001, η2p = 0·19; face likeness U = 16, p < 0·001, r = 0·82). In contrast, we did not observe a group difference with regard to the positive effects of being liked by others (F(0,47) = 180·3, p = 0·78, η2p = 0·002; Fig. 1).
Fig. 1.
Subjective perception of the social interaction as measured by post-scan questions. NSSI individuals were significantly different in all scores except for sensitivity to acceptance. * indicate p < 0·01, ** indicate p < 0·001.
We observed significant correlations between NSSI characteristics and behavioral data. The average cutting frequency, which reflected the number of cuts during the past 12 months was 54.6 (SD 55.7). One patient was an extreme outlier, with an estimated cutting frequency of 400, and was therefore removed from the analysis. Cutting frequency was negatively correlated with how much participants liked to see their own face (rs(23) = − 0·47, p = 0·024) and positively correlated to perceived rejection scores (rs(23) = 0·46, p = 0·026). Further, the recency of the latest NSSI episode was negatively correlated with perceived rejection, indicating that the more recent the NSSI episode, the more the patient felt rejected (rs(24) = − 0·48, p = 0·016).
The pattern and significance of results did not change when excluding NSSI patients on medication (see Supplemental data). Overall, our behavioral findings suggest that NSSI patients have a negative-interpretation bias for social evaluation, and when presented with negative feedback, are more sensitive to it than controls. This bias correlates with a critically important clinical parameter, the frequency of NSSI events.
3.2. Univariate Analysis: Brain Correlates to Self-relevant Evaluation
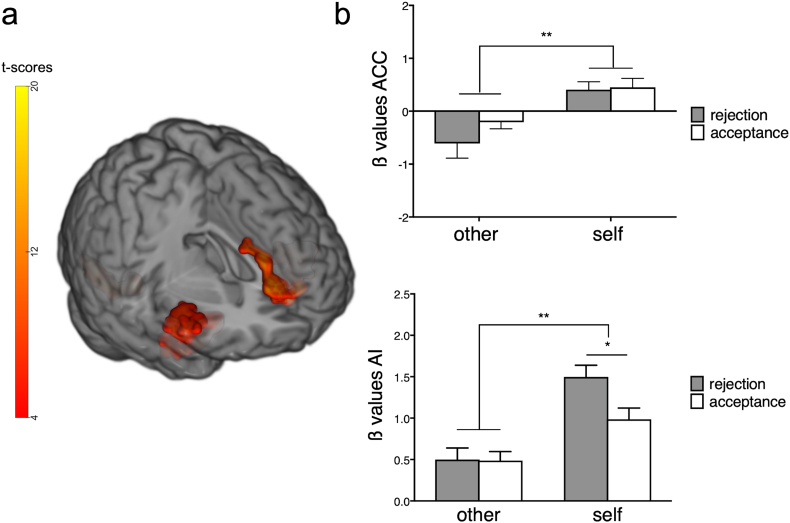
We previously reported that right anterior insula and dorsal anterior cingulate cortex (rAI and dACC) are key regions for processing self-relevant information in healthy adolescents [14]. These findings replicated in both healthy and NSSI individuals in the present study. For the main effect of Perspective, clusters in bilateral AI and ACC merging with orbitofrontal cortex, together with a cluster in the visual cortex were significantly activated (p < 0·002, family-wise-error corrected; Fig. 2a, Table 2). No between-group differences were identified in this analysis (Table 2). ß values within the right AI, showed a significant effect of Perspective with “self” condition values higher than “other” condition values [F(1,52) = 51·3, p < 0·001, η2p = 0·49]. A significant effect of outcome [F(1,52) = 8.73, p = 0·005, η2p = 0·14] driven by higher values for rejection (t = 2.9, p = 0·003) and a perspective-by-outcome interaction was observed [F(1,52) = 9·3, p = 0·003, η2p = ·15], with the self-rejection condition significantly higher than self-acceptance (t = 4·3, p < 0·001; Fig. 2b). ß values extracted from the ACC showed higher values for “self” compared to “other” condition [F(1,52) = 18·9, p < 0·001, η2p = 0·26; Fig. 2b].
Fig. 2.
Whole-brain GLM-based analysis results. (a). Significant rAI and ACC activations for the factor perspective (per-voxel p < 0·002, alpha = 0·05 family-wise error corrected). (b). Bar graphs show significantly higher β-values for “self” versus “other” conditions in ACC and AI. In rAI β-values, a perspective x perspective interaction was observed (p = 0·004), with significantly increased activity to self-rejection compared to self-acceptance. Error bars represent standard error of the mean. * indicate p < 0·01, ** indicate p < 0·001.
Table 2.
Activations associated with the whole-brain analyses during anticipation and outcome intervals, expressed by peak scores in Talairach-space coordinates (x, y, z). Z-scores survived significance threshold (p < 0·002, cluster corrected alpha < 0·05).
| Analysis | Regione | Talairach coordinates |
Voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Anticipation interval | |||||
| Self > other | Supplementary motor area (peak) | 2 | 2 | 53 | 231 |
| − 3 | 9 | 51 | |||
| Dorsal anterior cingulate cortex | 6 | 8 | 43 | ||
| − 4 | 7 | 43 | |||
| Postcentral gyrus | − 37 | − 37 | 38 | 42 | |
| − 44 | − 33 | 54 | |||
| Anterior insula | 35 | 17 | 8 | 93 | |
| − 31 | 14 | 11 | 69 | ||
| Outcome interval | |||||
| Self > other | Inferior frontal gyrus (peak) | 44 | 14 | − 10 | 210 |
| Right anterior insula | 31 | 18 | − 5 | ||
| Right mid-anterior insula | 41 | 9 | − 1 | ||
| Rostral anterior cingulate cortex (peak) | 2 | 35 | 11 | 46 | |
| − 1 | 36 | 13 | |||
| Dorsal anterior cingulate cortex | 2 | 15 | 26 | ||
| − 1 | 14 | 26 | |||
| Inferior frontal gyrus (peak) | − 28 | 11 | − 16 | 92 | |
| Left anterior insula | − 31 | 17 | − 5 | ||
| Left mid-anterior insula | − 37 | 11 | 5 | ||
| Fusiform gyrus | 29 | − 43 | − 13 | 39 | |
| Middle occipital gyrus | − 28 | − 64 | 2 | 69 | |
For the anticipation interval, the whole-brain, grey-matter analysis showed activation of the rAI, the dACC and supplementary motor area (SMA) bilaterally for the “self” versus “other” conditions contrast in both groups (p < 0·002, family-wise-error corrected; Fig. 3c; Table 2). Again, no significant between-group effects were observed (Table 2).
Fig. 3.
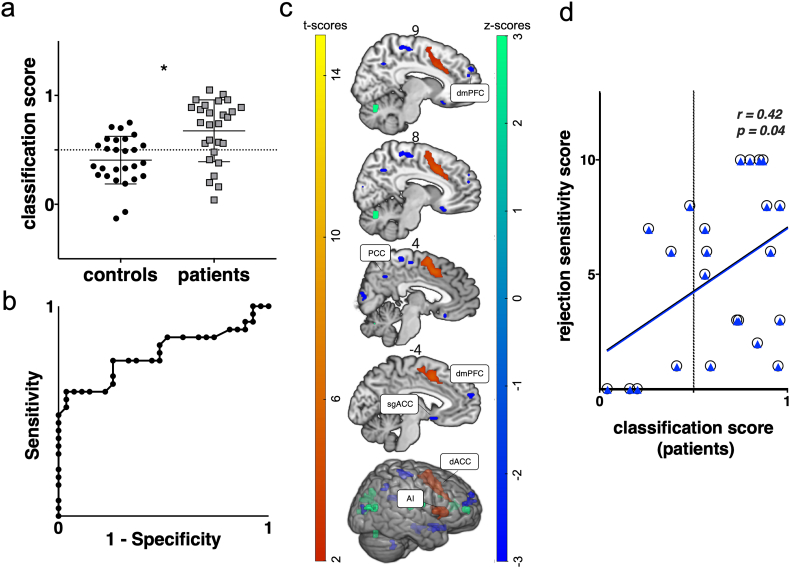
Multi-voxel pattern analysis results. (a) Support vector machine (svm) classification performance based on functional brain data during the anticipation interval. Accuracy = 0·68, permutation corrected p = 0·031. Sensitivity = 0·74 and specificity = 0·59. (b) ROC curve depicting classification performance (AUC = 0·77, p = 0·001). (c) GLM-based results showing common activity in both groups for the effect of “self” vs “other” during the anticipation interval (red-yellow). Weight vector map showing brain regions which contributed to the discrimination between groups during the anticipation interval (blue-green). Maps were thresholded at per-voxel p < 0·002, alpha = 0·05 family-wise error corrected. (d) Scatter plot depicting a significant correlation between classification and sensitivity to rejection scores in NSSI individuals. White circles denote classification scores from a whole-brain, grey matter mask. Blue triangles represent classification scores from a grey matter mask excluding the regions activated during the univariate analysis for the self-vs-other contrast.
3.3. Multivariate Analysis: Neural Response During Anticipation of Self-relevant Evaluation
The whole-brain, grey-matter, multivariate analysis rendered statistically-significant classification of subjects during anticipation, with accuracy of 68% (the percentage of patients that were correctly classified), sensitivity = 0·74, specificity = 0·59 (Fig. 3a); permutation p = 0·031; AUC ROC = 0·77, p = 0·001 (Fig. 3b). Classification scores remained significantly different between groups after excluding NSSI patients on medication (U = 117, p = 0·007, r = 0·32). Classification scores derived from the multivariate analysis, significantly correlated with rejection sensitivity scores in the NSSI patients but not in controls [patients rs(25) = 0·42, p = 0·04, controls rs(23) = 0·12, p = 0·57; r scores comparison not significant p = 0·1, two-tails) (Fig. 3d, circles)]. The correlation remained significant after controlling for BDP traits [rs(22) = 0·46, p = 0·02], depression traits [rs(20) = 0·47, p = 0·03], and medication use [rs(22) = 0·42, p = 0·04].
Brain regions significantly contributing to the discrimination between groups included dorsomedial prefrontal cortex (dmPFC; three clusters), posterior cingulate cortex (PCC) and subgenual anterior cingulate cortex (sgACC) (Fig. 3c; Table 3). Excluding from the analysis mask regions activated for the self-versus-other contrast during anticipation yielded the same outcome, with classification accuracy = 68% and permutation p = 0·029. Classification scores significantly correlated with rejection sensitivity [patients r(21) = 0·42, p = 0·04 (Fig. 3d, triangles)]. To investigate the composition of the multivariate effects we extracted ß-coefficients from the aforementioned regions and compared them between groups (see Fig. S1). Average-ß scores for all regions were significantly lower in NSSI [sgACC (U = 221, p = 0·005), PCC (U = 253, p = 0·02), dmPFC1 (U = 172, p < 0·001), dmPFC2 (U = 225, p = 0·006), dmPFC(U = 227, p = 0·007)] (Fig. S1).
Table 3.
Brain regions associated with the mvpa whole brain, grey matter, analysis during the anticipation interval, expressed in Talairach-space coordinates (x, y, z). Z-scores survived significance threshold (p < 0·002, cluster corrected alpha < 0·05).
| Analysis svm classification | Region | Talairach coordinates |
Voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Anticipation self | Superior/middle occipital gyrus | − 31 | − 76 | 26 | 40 |
| Middle temporal gyrus | − 52 | − 16 | − 13 | 31 | |
| Dorsolateral prefrontal cortex | − 49 | 38 | 14 | 29 | |
| Insula | − 43 | − 37 | 20 | 28 | |
| Paracentral lobule | 8 | − 34 | 56 | 23 | |
| Superior temporal gyrus | − 46 | − 64 | 17 | 18 | |
| Subgenual anterior cingulate cortexa | − 4 | 11 | − 10 | 16 | |
| Dorsomedial prefrontal cortexa | 23 | 50 | 17 | 16 | |
| Dorsomedial prefrontal cortexa | − 4 | 56 | 17 | 11 | |
| Dorsomedial prefrontal cortexa | 11 | 56 | 26 | 8 | |
| Middle occipital gyrus | − 37 | − 79 | 5 | 16 | |
| Precuneus | − 25 | − 67 | 29 | 14 | |
| Ventrolateral prefrontal cortex | 29 | 47 | 11 | 13 | |
| Middle temporal gyrus | 41 | − 76 | 11 | 13 | |
| Postcentral gyrus | − 37 | − 31 | 56 | 13 | |
| Orbitofrontal cortex | 23 | 11 | − 13 | 13 | |
| Orbitofrontal cortex | − 25 | 17 | − 13 | 11 | |
| Orbitofrontal cortex | − 22 | 8 | − 13 | 8 | |
| Orbitofrontal cortex | 8 | 20 | − 13 | 6 | |
| Posterior cingulate cortexa | 8 | − 55 | 35 | 12 | |
| Ventral cuneus | 2 | − 82 | 11 | 11 | |
| Cerebellum pyramis | 8 | − 67 | − 25 | 11 | |
| Cerebellum lobule IX | − 13 | − 43 | − 37 | 11 | |
| Dorsolateral prefrontal cortex | − 37 | 20 | 32 | 8 | |
| Right parietal operculum (OP4) | 59 | − 4 | 17 | 6 | |
| Insula | 35 | − 22 | 11 | 6 | |
| Dorsolateral prefrontal cortex | 41 | 26 | 26 | 6 | |
Indicate regions for which average ß-values were extracted during the anticipation period (see Fig. S1).
The multivariate analyses targeting the outcome intervals did not yield above-chance classification accuracy scores (outcome self-rejection accuracy = 46%, outcome self-acceptance accuracy = 31%).
3.4. Facial EMG Findings
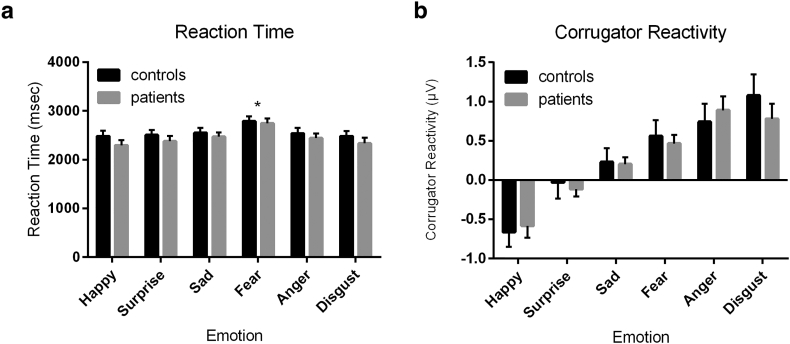
Behaviorally, there was a main effect of emotion on reaction time (F(5,270) = 11·7, p < 0·001) and accuracy (F(5,285) = 29·2, p < 0·001), but this did not differ between patients and controls (Reaction time: group, p = 0·37; group*emotion, p = 0·83; Accuracy: group, p = 0·38; emotion* group, p = 0·32). Specifically, all participants were less sensitive (e.g. slower) and accurate to detect fear (p < 0·05 versus other emotion, Bonferroni corrected).
There was a main effect of emotion on corrugator reactivity such that faces conveying negative emotions (fear, anger, disgust) elicited an increase in corrugator reactivity, while happy faces elicited a reduction in activity (F(5,275) = 26·9, p < 0·001). There was no effect of group (p = 0·76) or a group-by-emotion interaction (p = 0·80). Similarly, happy faces elicited significantly more zygomatic activity than surprise or the negative emotions (F(5,275) = 5·99, p < 0·001), but these findings were consistent across patients and controls (group, p = 0·95; emotion*group, p = 0·17).
When including sensitivity (e.g. reaction time) in the analysis, we still found no effect of group on corrugator reactivity (happy: p = 0·73; surprise: p = 0·74; sad: p = 0·92; fear: p = 0·78; anger: p = 0·43; disgust: p = 0·57) or zygomatic reactivity (happy: p = 0·35; surprise: p = 0·30; sad: p = 0·22; fear: p = 0·27; anger: p = 0·82; disgust: p = 0·32).
4. Discussion
This study is to our knowledge the first to examine neural functioning during a simulated online interaction in NSSI CAP patients. We assessed the largest sample of adolescents with NSSI up to date, both behaviorally and neurally, to characterize processing of social stimuli. We found that adolescents with NSSI showed a negative bias in interpreting evaluative social feedback from others. We found that this bias was associated with distinct neural-response patterns to social evaluation, as identified by multivoxel pattern analysis. The clinical relevance of the negative social-evaluation bias we observed is indicated by its correlation with the time since the most recent NSSI episode. The clinical relevance of the neural-response patterns to NSSI is suggested by the significant correlation between classifier scores and rejection sensitivity.
NSSI is a substantial clinical problem, overrepresented in adolescent and young adult females [4], and remains the cause of considerable disability in a subgroup as they become adults. There is a lack of diagnostic and predictive biomarkers in NSSI that could be applied to this disorder. NSSI can occur with and independently of several psychiatric diagnoses; when studied in groups recruited on the basis of psychiatric disorders with comorbid NSSI, the relationship of findings to NSSI can be difficult to determine. Thus, a strength of our study is the use of NSSI as the primary inclusion criterion.
The ability to correctly read evaluative feedback from others during social interactions is important for successfully navigating them, and maintaining healthy relationships. The hypothesis that processing of social evaluation is altered in NSSI patients stems from the observation that interpersonal stress can trigger NSSI [12]. In the present study, we find support for this notion using a paradigm previously tested in healthy adolescents, which demonstrated at a neural level the selective salience of self-referential social evaluation-stimuli [14]. Mimicking popular social media platforms, this task uses simple feedback in the form of a thumbs up/thumbs down to convey positive and negative feedback. The NSSI group showed a significant negative bias in interpreting feedback. This finding both confirms prior clinical observation [33] and does so under controlled, ecologically valid experimental conditions. Interpersonal stressors, such as those used here, may enhance negative affect, potentially promoting NSSI as an emotion regulation strategy [1].
Social media platforms are an increasingly common means of interacting with others. However, scientists only recently started to address the role of social media experimentally, focusing mostly on the rewarding value of positive feedback from others [34], [35]. Our social interaction task [14] was specifically designed to be balanced in terms of affective valence, including an equal number of positive and negative feedback events. This balance allowed us to identify a negative evaluative bias in the NSSI patients not contaminated by effects of frequency of exposure. In other words, although persons with NSSI received the same number of thumbs up/down, they felt more rejected by others than controls. In addition, they felt worse when rejected by others compared to controls, suggesting higher rejection sensitivity.
Previous research shows that adolescents with NSSI report more experiences of interpersonal stress and aversive life-events than individuals who do not exhibit self-injury behaviors [18]. The sensitivity to rejection found in our study could potentially be viewed as part of a transactional process involving vulnerability and stress [33]. Adolescents' interpersonal experience are most likely relevant for understanding and treating adolescent NSSI, and treatment would benefit from addressing social information processing [33].
The chain of processes invoked by the task we employed is complex and involves perception and evaluation of facial stimuli and others' reactions to these stimuli. Using facial EMG, we excluded the possibility that behavioral differences observed between NSSI and healthy participants were due to altered facial expression processing. We used a dynamically developing emotion task to assess sensitivity to detecting emotions and accuracy in identifying emotions conveyed. Dynamic tasks are likely more ecologically valid to assess emotion processing, as they engage brain areas involved in emotion processing to a greater extent than tasks using static images [36]. These methodological differences may explain why previous studies [37] reported deficits in emotion identification associated with NSSI using static emotion images. Using the dynamic task, we found that patients did not differ in their sensitivity to detecting and identifying emotions. Moreover, we found no difference in emotional reactivity to emotional faces as assessed via facial EMG (Fig. 4). This suggests that our neuroimaging results cannot be explained as a non-specific negativity bias in response to emotionally ambiguous faces [38].
Fig. 4.
No differences in identification of or reaction to emotional faces. (a) All individuals were slower at identifying fear as compared to other emotions, but there was no difference in reaction times between groups. (b) While the emotional faces elicited distinct corrugator reactivity, there again was no difference between controls and patients. Thus, patients and controls do not differ in their sensitivity to detect emotions, nor in their affective reactions to emotional faces.
Using a GLM approach, we previously identified BOLD-response correlates of processing self-relevant information during a social interaction [14]. AI and dACC, key nodes of the salience network, were significantly more active when healthy participants were judged by others, independently of the quality of the feedback. Interestingly, these areas were active during both the anticipation of judgment and during judgment, itself. Here, we robustly replicated this finding. However, no group difference in processing of self-relevant information was present within these brain areas.
A univariate approach can fail to identify potentially relevant information from spatially distributed effects [39]. Using a multivariate approach, we identified a pattern of brain regions in which activity during anticipation of judgment by others robustly classified subjects into patient and control groups. Most interestingly, the identified regions, which included—sgACC, dmPFC and PCC are outside the salience network. The sgACC is considered to be involved in the generation of affective states and has been shown to be anatomically and functionally altered in psychiatric conditions, in particular in mood disorders [40]. The other regions, which include PCC and portions of dmPFC have been shown to be involved with self-referential processing and autobiographical memory [41]. A possible interpretation of the pattern that emerges is that attribution of salience to the self-referential social feedback stimuli by the ACC and insular cortex is not altered in NSSI, but that activity of other brain areas subsequently results in a negative interpretation bias in the patient group.
The validity and clinical relevance of our multi-variate approach is supported by the finding that multivariate classification scores correlated significantly with rejection sensitivity, suggesting a potential neurobiological basis for the reported behavioral differences in response to social feedback. Importantly, this correlation remained significant after controlling for symptoms of BPD, a condition in which great efforts are made to avoid perceived or real abandonment [6], and also after controlling for symptoms of depression, where negative self-evaluation is pronounced [6]. In our view, this finding provides preliminary support for the validity and utility of NSSI as an independent diagnostic entity.
There are limitations to our study. As is the case in all cross-sectional fMRI studies, our ability to infer a causal relationship between any of the brain responses and the behavioral findings is limited. Finally, because our finding is the first of its kind, our data need to be replicated in an independent sample. Nevertheless, our results have several potentially important clinical implications. Although the identified behavioral results are task-specific and were not investigated using a standardized self-report, they point to a vulnerability and negative affective bias that requires emphasis in clinical practice. Treatment approaches that make adolescents with NSSI aware of the existence of their affective biases can help making social interactions less painful, and promote more favorable interpretations of social feedback, thus reducing a potential trigger of NSSI. Some of the measures employed in our study have the potential to serve as biomarkers of therapeutic response to interventions, and future research should be directed to exploring this potential.
In summary, this study used a simulation of online social interaction to provide novel insight into the behavioral and neurological mechanisms of NSSI. The findings from this ecologically valid paradigm can potentially advance effective treatment, such as behavioral training using multivariate real-time fMRI neurofeedback.
Author Contributions
I.P., M.Z., P.G., M.H. designed research; I.P., R.K., M.Z., L.M. performed research; I.P., R.K. P.H., L.M. analyzed data; I.P. made figures; I.P., P.H., R.K., M.Z., L.M., P.G, M.H., wrote the paper.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
The Authors would like to thank professor Martin Paulus for thoughtful contribution to the study design. This research was supported by The Swedish Research Council (538-2013-7434) and the ALF Grants, Östergötland County (LIO-535931; LIO-520131). The authors gratefully acknowledge staff at the Center for Medical Imaging and Visualization (CMIV) and the Child- and Adolescent Psychiatric clinic, Linköping University Hospital, Sweden.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.06.016.
Appendix A. Supplementary Data
Supplementary material
References
- 1.Nock M.K. Self-injury. Annu Rev Clin Psychol. 2010;6:339–363. doi: 10.1146/annurev.clinpsy.121208.131258. [DOI] [PubMed] [Google Scholar]
- 2.Muehlenkamp J.J., Claes L., Havertape L., Plener P.L. International prevalence of adolescent non-suicidal self-injury and deliberate self-harm. Child Adolesc Psychiatry Ment Health. 2012;6:10. doi: 10.1186/1753-2000-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klonsky E.D., Muehlenkamp J.J. Self-injury: a research review for the practitioner. J Clin Psychol. 2007;63(11):1045–1056. doi: 10.1002/jclp.20412. [DOI] [PubMed] [Google Scholar]
- 4.Bresin K., Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55–64. doi: 10.1016/j.cpr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Kiekens G.A.-O., Hasking P., Claes L. The DSM-5 nonsuicidal self-injury disorder among incoming college students: prevalence and associations with 12-month mental disorders and suicidal thoughts and behaviors. Depress Anxiety. 2018;35(7):629–637. doi: 10.1002/da.22754. [DOI] [PubMed] [Google Scholar]
- 6.APA . 5th ed. American Psychiatric Publishing; Washington DC: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 7.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Moran P., Coffey C., Fau-Romaniuk H., Romaniuk H., Fau-Olsson C. The natural history of self-harm from adolescence to young adulthood: a population-based cohort study. Lancet. 2012;379:236–243. doi: 10.1016/S0140-6736(11)61141-0. [DOI] [PubMed] [Google Scholar]
- 9.Miller A.B., Linthicum K.P., Helms S.W. Reciprocal associations between adolescent girls' chronic interpersonal stress and nonsuicidal self-injury: a multi-wave prospective investigation. J Adolesc Health. 2018;63:694–700. doi: 10.1016/j.jadohealth.2018.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K.L., Cushman G.K., Weissman A.B. Behavioral and emotional responses to interpersonal stress: a comparison of adolescents engaged in non-suicidal self-injury to adolescent suicide attempters. Psychiatry Res. 2015;228:899–906. doi: 10.1016/j.psychres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Santangelo P.S., Koenig J., Funke V. Ecological momentary assessment of affective and interpersonal instability in adolescent non-suicidal self-injury. J Abnorm Child Psychol. 2017;45:1429–1438. doi: 10.1007/s10802-016-0249-2. [DOI] [PubMed] [Google Scholar]
- 12.Nock M.K., Prinstein M.J., Sterba S.K. Revealing the form and function of self-injurious thoughts and behaviors: a real-time ecological assessment study among adolescents and young adults. J Abnorm Psychol. 2009;118:816–827. doi: 10.1037/a0016948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown R.C., Plener P.L., Groen G., Neff D., Bonenberger M., Abler B. Differential neural processing of social exclusion and inclusion in adolescents with non-suicidal self-injury and young adults with borderline personality disorder. Front Psych. 2017;8:267. doi: 10.3389/fpsyt.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perini I., Gustafsson P.A., Hamilton J.P., Kampe R., Zetterqvist M., Heilig M. The salience of self, not social pain, is encoded by dorsal anterior cingulate and insula. Sci Rep. 2018;8:6165. doi: 10.1038/s41598-018-24658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordicom-Sveriges . 2017. Mediebarometer. Gothenburg. [Google Scholar]
- 16.Kaufman J., Birmaher B., Fau-Brent D., Brent D., Fau-Rao U. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Nock M.K., Holmberg Eb Fau-Photos V.I., Photos Vi Fau-Michel B.D., Michel B.D. Self-injurious thoughts and behaviors interview: development, reliability, and validity in an adolescent sample. Psychol Assess. 2007;19:309–317. doi: 10.1037/1040-3590.19.3.309. [DOI] [PubMed] [Google Scholar]
- 18.Zetterqvist M., Lundh Lg Fau-Svedin C.G., Svedin C.G. A comparison of adolescents engaging in self-injurious behaviors with and without suicidal intent: self-reported experiences of adverse life events and trauma symptoms. J Youth Adolesc. 2013;42:1257–1273. doi: 10.1007/s10964-012-9872-6. [DOI] [PubMed] [Google Scholar]
- 19.First M.B., Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S. American Psychiatric Press, Inc; Washington, D.C.: 1997. Structured clinical interview for DSM-IV axis II personality disorders, (SCID-II) [Google Scholar]
- 20.Poznanski E., Mokros H. WPS; Los Angeles: 1996. Children's depression rating scale–revised (CDRS-R) [Google Scholar]
- 21.Wechsler D. 4th ed. Pearson; London: 2003. The Wechsler intelligence scale for children. [Google Scholar]
- 22.Wechsler D. 4th ed. Pearson; London: 2008. The Wechsler adult intelligence scale. [Google Scholar]
- 23.Gratz K.L., Dixon-Gordon K.L., Chapman A.L., Tull M.T. Diagnosis and characterization of DSM-5 nonsuicidal self-injury disorder using the clinician-administered nonsuicidal self-injury disorder index. Assessment. 2015;22:527–539. doi: 10.1177/1073191114565878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 25.Talairach J., Tournoux P. Thieme Medical Publishers; New York: 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- 26.Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7(3):152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. fMRI clustering and false-positive rates. Proc Natl Acad Sci U S A. 2017;114(17):E3370-E1. doi: 10.1073/pnas.1614961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bamford S., Penton-Voak I., Pinkney V., Baldwin D.S., Munafo M.R., Garner M. Early effects of duloxetine on emotion recognition in healthy volunteers. J Psychopharmacol (Oxford, England) 2015;29(5):634–641. doi: 10.1177/0269881115570085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiddeman B., Burt M., Perrett D. Prototyping and transforming facial textures for perception research. IEEE Comput Graph Appl. 2001;21(5):42–50. [Google Scholar]
- 30.Skinner A.L., Benton C.P. Anti-expression aftereffects reveal prototype-referenced coding of facial expressions. Psychol Sci. 2010;21(9):1248–1253. doi: 10.1177/0956797610380702. [DOI] [PubMed] [Google Scholar]
- 31.Mayo L.M., de Wit H. Acquisition of conditioned responses to a novel alcohol-paired cue in social drinkers. J Stud Alcohol Drugs. 2016;77(2):317–326. doi: 10.15288/jsad.2016.77.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wardle M.C., de Wit H., Penton-Voak I., Lewis G., Munafo M.R. Lack of association between COMT and working memory in a population-based cohort of healthy young adults. Neuropsychopharmacology. 2013;38:1253–1263. doi: 10.1038/npp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prinstein M.J., Guerry J.D., Browne C.B., Rancourt D. Interpersonal models of nonsuicidal self-injury. In: Nock M.K., editor. Understanding nonsuicidal self-injury origins, assessment, and treatment. American Psychological Association; Washington D.C.: 2009. pp. 79–98. [Google Scholar]
- 34.Sherman L.E., Hernandez L.M., Greenfield P.M., Dapretto M. vol. 13. 2018. What the brain ‘likes’: neural correlates of providing feedback on social media; pp. 669–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman L.E., Payton A.A., Hernandez L.M., Greenfield P.M., Dapretto M. The power of the like in adolescence: effects of peer influence on neural and behavioral responses to social media. Psychol Sci. 2016;27(7):1027–1035. doi: 10.1177/0956797616645673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaBar K.S., Crupain Mj Fau-Voyvodic J.T., Voyvodic Jt Fau-McCarthy G., McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cereb Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- 37.Seymour K.E., Jones R.N., Cushman G.K. Emotional face recognition in adolescent suicide attempters and adolescents engaging in non-suicidal self-injury. Eur Child Adolesc Psychiatry. 2016;25:247–259. doi: 10.1007/s00787-015-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neta M., Norris Cj Fau-Whalen P.J., Whalen P.J. Corrugator muscle responses are associated with individual differences in positivity-negativity bias. 2009;9:640–648. doi: 10.1037/a0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox D.D., Savoy R.L. Functional magnetic resonance imaging (fMRI) "brain reading": detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage. 2003;19:261–270. doi: 10.1016/s1053-8119(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 40.Drevets W.C., Savitz J., Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spreng R.N., Mar Ra Fau-Kim A.S.N., Kim A.S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. 2008;3:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material