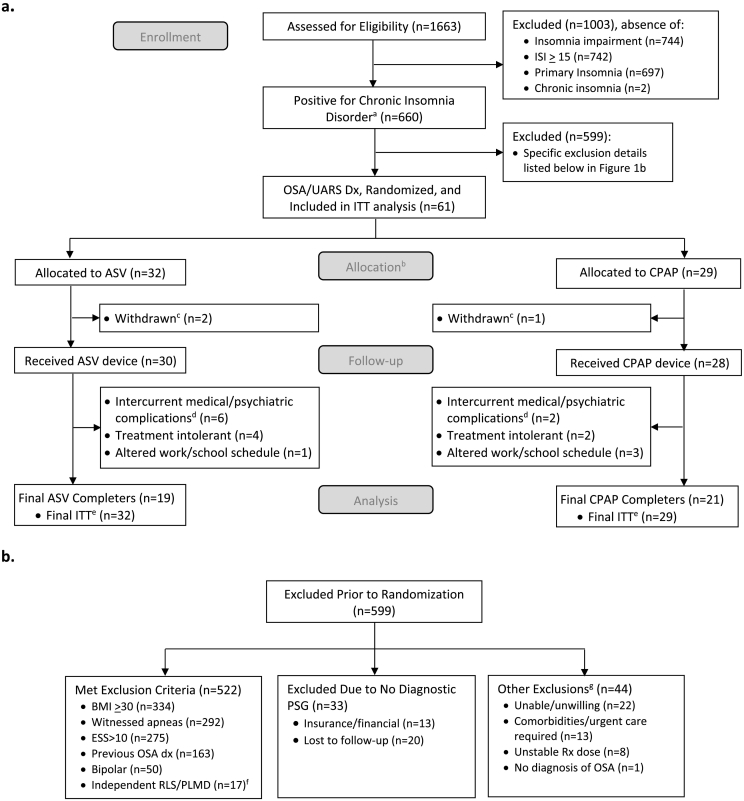
Fig. 1.
Flowchart showing a) eligibility and final sample of chronic insomnia disorder patients presenting with chief complaint of chronic psychophysiological insomnia attributed to behavioral, psychological, psychiatric, and environmental factors, and b) excluded patients' details.
Abbreviations: ISI — Insomnia Severity Index; ITT — intent to treat; Dx — diagnosis; BMI — body mass index; ESS — Epworth Sleepiness Scale; PSG — Polysomnography Sleep Test; Rx — prescription; OSA — obstructive sleep apnea; UARS — upper airway resistance syndrome; ASV — adaptive servo-ventilation; CPAP — continuous positive airway pressure; RLS — restless leg syndrome; PLMD — periodic limb movement disorder.
Footnote:
aChronic insomnia disorder as defined by the AASM: chief complaint of insomnia, ISI ≥ 15, impairment due to insomnia, and duration of at least 6 months.
bRandomization reset halfway through sample collection due to exclusion of ASV participants secondary to disproportionate leg movement disorders in this group.
cWithdrawn patients after randomization and completion of first exposure to PAP treatment; chose not to pursue research without offering explanation.
dIntercurrent medical/psychiatric patients developed medical or psychiatric issues that required immediate attention and subsequent removal from the study due to treatment plan.
eFinal ITT included all eligible randomized patients, total n = 61.
fThe 17 exclusions were eligible for the study and completed the informed consent and randomization process. However, during a titration study they exhibited independent RLS/PLMD and were excluded as post-randomization exclusions.
gUnable/unwilling: patients with scheduling conflicts or no interest in participation; comorbidities/urgent care required: patients with multiple health issues that may have confounded research or patients who needed immediate treatment of their sleep-disordered breathing; unstable Rx dose: patients working with doctors to titrate medication dosage; no diagnosis of OSA: did not meet requirements for OSA/UARS.