Abstract
Bilateral oophorectomy in premenopausal women is a unique condition causing the abrupt and premature loss of ovarian hormones, primarily estrogen. Bilateral oophorectomy causes an alteration of several fundamental aging processes at the cellular, tissue, organ, and system levels, leading to multimorbidity, frailty, and reduced survival. However, many questions remain unanswered.
Introduction
Within the evolutionary framework, cessation or diminution of reproductive capacity is a key manifestation of aging, and sex hormones are potent mediators of the homeostasis of the entire body (21). Thus the perturbation of naturally occurring sex hormones may accelerate the age-related dysfunction of tissues and may cause subsequent decline in physical and cognitive health. This review will focus on animal studies and clinical studies that show that the premature and abrupt disruption of ovarian function accelerates the aging processes, causing an accelerated decline in physical and cognitive functions. Current controversies in the field regarding potential mechanisms mediating these effects will be discussed along with the potential clinical implications for the health of an aging population.
Sex Steroid Hormones
Estrogen is the primary sex hormone in female mammals, and the ovaries are the principal source of estrogen. However, non-gonadal tissues (e.g., breast, brain, and adipose tissue) also produce low levels of estrogen, with predominantly local activity (paracrine or intracrine activity). In postmenopausal women, the peripheral aromatization of androgens in the adipose tissue may produce sizable amounts of circulating estrogen (usually estrone). The ovaries also produce other important sex steroid hormones, including progesterone and testosterone (42).
In addition to its powerful roles in regulating the development and homeostasis of reproductive tissues, estrogen provides critical signaling and trophic support to a range of tissues throughout the entire body and across the lifespan. Indeed, estrogen signaling influences all systems of the body through activation of estrogen receptors, ERα, ERß, and the G-protein-coupled estrogen receptor (GPER; also known as GPR30) (FIGURE 1) (18, 26, 29, 48). The distribution of these receptors varies among tissues, and thus estrogen determines tissue-specific responses (18, 26, 29, 48).
FIGURE 1.
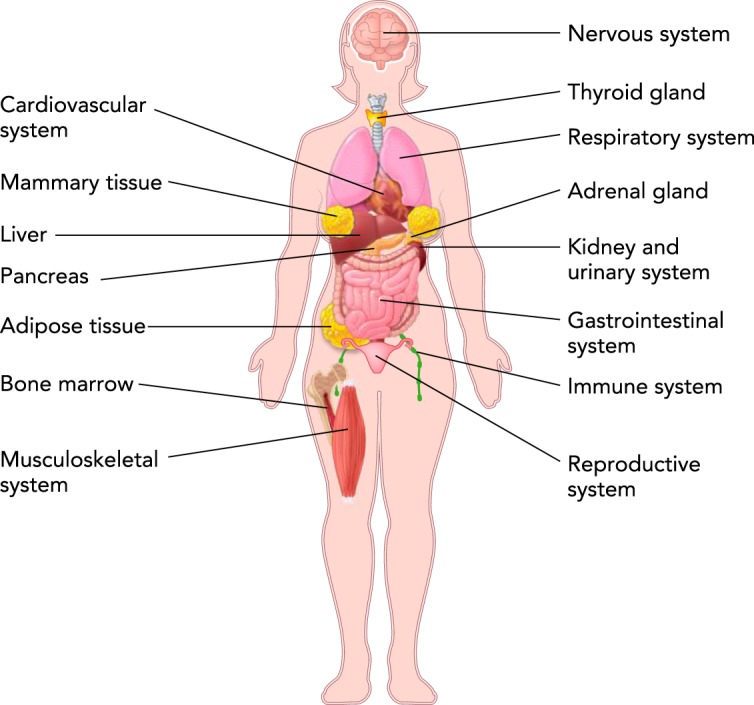
Illustration of the most important organs and systems under the influence of estrogen in women
For example, in the cardiovascular system, estrogen upregulates nitric oxide production to enhance endothelium-dependent vascular function (4) and regulates cytokine-mediated cell adhesion and anti-atherogenic activity (3, 6). In the brain, estrogen suppresses microglial and astrocyte-dependent immune activation and neuroinflammation (2). In addition, estrogen influences the generation, maintenance, and remodeling of synaptic connections (14, 23, 41). In muscle, estrogen influences satellite cell activation and proliferation, benefiting muscle maintenance and restorative capacity (20). Estrogen also regulates mitochondrial metabolism and insulin sensitivity in muscle (46). Some effects of estrogen in diverse tissues, including brain, muscle, and vasculature, have been attributed to its ability to reduce oxidative stress (1, 8, 25). Therefore, due to the diverse cellular and molecular targets of estrogen, the reduction of endogenous production during menopause or caused by the premature removal of the ovaries may accelerate the mechanisms of cellular aging, leading to increased risk of age-related conditions and reduced quality of life, a phenomenon described as a reduction in health span (30, 31, 33).
Bilateral Oophorectomy
Animal Studies
A large body of literature from animal experiments supports the detrimental effects of ovariectomy on several aging processes and on overall health and lifespan. Bilateral ovariectomy in young mammals is the experimental intervention used routinely to study the effects of premature estrogen deprivation on specific tissues or organs (e.g., heart, brain, bone) and to study the effects of estrogen or of selective estrogen receptor agonists or antagonists as therapeutic interventions.
Studies in monkeys showed an acceleration of vascular degeneration following ovariectomy (7, 19). Other studies showed an acceleration of brain degeneration following ovariectomy in rodents and monkeys (13, 14). Studies in female dogs and female mice showed a reduction in lifespan following ovariectomy (5, 49). Finally, transplantation of the ovaries from young mice into old female mice was shown to increase lifespan significantly (24), thus supporting the general hypothesis that ovarian function affects the aging processes and health span.
Clinical and Epidemiological Studies
Women who underwent oophorectomy for the prophylaxis of breast and ovarian cancer in previous decades provide a unique natural experiment to study the effect of endocrine function in regulating the pace of aging. The degree of multimorbidity (number of chronic conditions compared with age- and sex-matched peers) has been proposed as a clinical marker of acceleration of the aging processes (9, 10, 44, 47). Thus a woman who has accumulated more chronic conditions than her age-matched peers is considered to have experienced accelerated aging. Indeed, multimorbidity mirrors a global susceptibility and loss of resilience, which are both hallmarks of aging (47). A related but distinct concept is frailty, defined as a condition of diminished strength, endurance, and reduced physiological function, increasing an individual’s vulnerability to dependency and death. Multimorbidity and frailty are two complementary measures of biological aging, but they should be considered separately because patients with multimorbidity may or may not be frail, and vice versa (47).
In the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2), we studied the association between bilateral oophorectomy performed before natural menopause and the accumulation of multimorbidity (33). We used the Rochester Epidemiology Project (REP) records-linkage system to identify all premenopausal women who underwent bilateral oophorectomy for a benign indication before age 50 between 1988 and 2007 in Olmsted County, MN. Each woman was randomly matched to a referent woman born in the same year (±1 yr) who had not undergone bilateral oophorectomy. We studied the rate of accumulation of 18 common chronic conditions over a median of 14 yr of follow-up. Inverse probability weights were used to balance the distributions at baseline in the oophorectomy and the referent cohorts for 18 chronic conditions, race/ethnicity, education, body mass index, smoking, age, and calendar year. This statistical method is a powerful way to bring observational studies closer in interpretation to randomized clinical trials when the exposure (in this case, bilateral oophorectomy) cannot be feasibly or ethically randomized (15, 38).
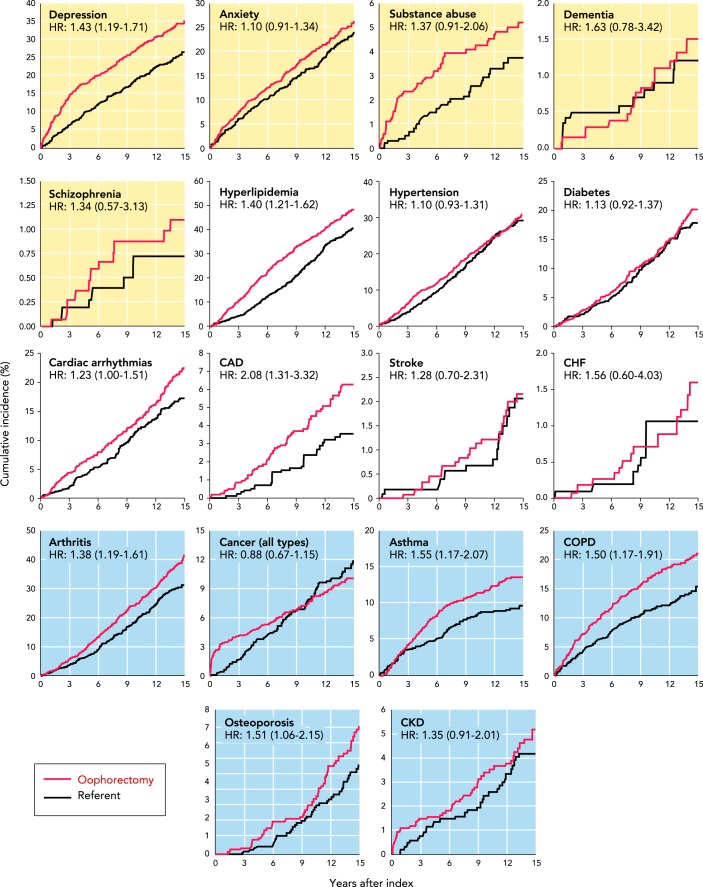
FIGURE 2 provides a visual summary of the results across multiple organs and systems in the subgroup of MOA-2 women who underwent oophorectomy before the age of 46 yr (premature or early surgical menopause). The measure of association [hazard ratio (HR)] was statistically significant for depression, hyperlipidemia, cardiac arrhythmias, coronary artery disease, arthritis, asthma, chronic obstructive pulmonary disease, and osteoporosis (8 of 18 conditions). The HR for cancer was not statistically significant, even though the bilateral oophorectomy had been performed with the intent of reducing the risk of cancer. Several of these associations were partly reduced in women who received systemic estrogen therapy to age 46 yr (ET; data not shown) (33). In addition, the women who underwent bilateral oophorectomy before the age of 46 yr experienced an accelerated rate of accumulation of the 18 chronic conditions considered together as a multimorbidity score (HR = 1.22; 95% confidence interval, 1.14–1.31; P < .001; data not shown) (33). Again, the association was stronger in women who did not receive ET to age 46 yr (data not shown) (33). The findings remained similar after removing from the study the women who had undergone hysterectomy (in both cohorts) or had reached menopause (only referent women) before the index day (33).
FIGURE 2.
Cumulative incidence of 18 chronic conditions considered separately in women with and without bilateral oophorectomy
Cumulative incidence of 18 chronic conditions considered separately in women with (red line) and without bilateral oophorectomy (black line). The analyses were restricted to women who underwent bilateral oophorectomy before age 46. The 18 graphs are grouped in 5 mental health conditions (yellow background), 7 cardiovascular or metabolic conditions (white background), and 6 other somatic conditions (blue background). The curves were adjusted using inverse probability weights derived from a logistic regression model including all 18 chronic conditions present at baseline, years of education (≤12, 13–16, >16), race (white vs. nonwhite), body mass index (<30 kg/m2 vs. ≥30 kg/m2), cigarette smoking (current or former vs. never), and age and calendar year at baseline (continuous). The number of women at risk varied across conditions because we excluded women with that specific condition on the index date. The HRs and corresponding 95% confidence intervals were calculated using Cox proportional hazards models, with age as the time scale and adjusted using inverse probability weights. Note the different scales used for the y-axis to better show differences. Therefore, the magnitude of the differences cannot be compared visually across conditions (e.g., schizophrenia vs. hyperlipidemia). CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
We are aware of only one additional study investigating the link between bilateral oophorectomy and measures of frailty. A 2018 Australian study showed an association between hysterectomy with bilateral oophorectomy and an increased risk of de novo physical function limitations that were self-reported using a recognized scale (51). Additional studies are needed in this important area of aging research.
Possible Explanations of the Findings
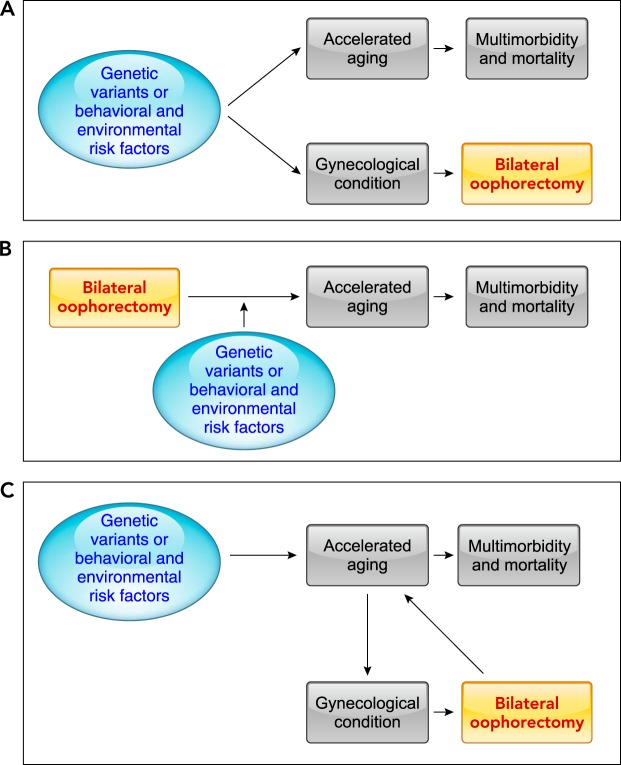
The results of the MOA-2 study confirmed what was expected from the animal and cellular studies that evaluated the mechanisms of estrogen action. However, a simple cause-effect model may not be the only explanation of the findings (FIGURE 3). First, our findings may reflect confounding by a shared genetic predisposition or by shared social or environmental risk factors that occurred early in life (FIGURE 3A). Genetic variants or behavioral and environmental risk factors may increase the risk of gynecologic diseases prompting the oophorectomy (e.g., ovarian cysts or endometriosis) or prompting hysterectomy accompanied by oophorectomy (e.g., uterine fibroids) (43, 50). These same genetic variants or behavioral and environmental risk factors may also independently increase the risk of multimorbidity (37) or of accelerated aging (21). As an example, women who are overweight or obese may have a higher risk of conditions prompting bilateral oophorectomy and multimorbidity (17, 33). In this scenario, a change in gynecological practice favoring ovarian conservation would not prevent the accumulation of multimorbidity and other accompanying adverse health outcomes. However, some of the findings from the MOA-2 study do not support this confounding mechanism. The primary analyses of the accumulation of multimorbidity adjusted for the 18 conditions present at baseline and for race/ethnicity, education, body mass index, smoking, age, and calendar year using inverse probability weights did not support a confounding mechanism (33). Similarly, sensitivity analyses restricted to women free of any condition at baseline and adjusted for the same potential confounders did not support a confounding mechanism (33). In addition, analyses stratified in women with or without a benign ovarian indication for the bilateral oophorectomy (e.g., cyst or endometriosis) did not reveal a significant difference (no confounding by indication).
FIGURE 3.
Possible interpretations of the findings
A: genetic variants in estrogen synthesis or responsiveness pathways, or behavioral and environmental risk factors are the true causes of both accelerated aging, leading to multimorbidity and increased mortality, and of the gynecological conditions prompting hysterectomy with bilateral oophorectomy. In this scenario, the oophorectomy is completely innocent and does not have any effect on aging. This scenario is called confounding in the epidemiological literature. B: bilateral oophorectomy has a direct causal effect on accelerated aging, leading to multimorbidity and increased mortality. In this scenario, genetic variants in estrogen synthesis or responsiveness pathways or behavioral and environmental risk factors may be effect modifiers of the causal pathway; however, oophorectomy is the major driver of the harmful effects. C: this is a more complex scenario in which bilateral oophorectomy is linked to accelerated aging both via a confounding mechanism and via a genuine causal effect. Even though genetic variants in estrogen synthesis or responsiveness pathways, or behavioral and environmental risk factors are the true causes of the preexisting accelerated aging manifesting as gynecological conditions that prompted hysterectomy with bilateral oophorectomy, the oophorectomy does have an incremental biological effect on the aging processes further accelerating a preexisting abnormal aging condition.
Second, the findings in MOA-2 may suggest that the premature and abrupt loss of estrogen, or other ovarian hormones, negatively affects multiple fundamental aging mechanisms, leading to harmful effects in multiple cells, tissues, organs, and systems (accelerated aging; FIGURE 3B). In this scenario, genetic variants or behavioral and environmental risk factors may have a modifying role by either facilitating or mitigating the effects of the abrupt loss of ovarian hormones (synergistic or antagonistic interactions) (28). Our findings may also suggest either protective effects of ovarian hormones other than estrogen (e.g., progesterone, testosterone, or inhibin) or harmful effects of the disruption of the hypothalamus-pituitary-ovarian (HPO) axis caused by bilateral oophorectomy (e.g., increased release of follicle-stimulating hormone and luteinizing hormone). However, the possible effects of these hormones on the cellular processes associated with aging remain unknown (37). In this scenario, a change in gynecological practice favoring ovarian conservation would reduce the accelerated accumulation of multimorbidity and other accompanying adverse health outcomes.
Third, it is possible that bilateral oophorectomy is partly a consequence of a preexisting condition of accelerated aging, but it is also a cause of a further acceleration of the aging processes (FIGURE 3C). In this scenario, a change in gynecological practice favoring ovarian conservation would reduce only in part the accelerated accumulation of multimorbidity and other accompanying adverse health outcomes. Findings in the MOA-2 study support this third mechanism at least for a subset of women. Approximately 59% of women who underwent bilateral oophorectomy before age 46 yr had one or more of the 18 chronic conditions used to define multimorbidity at the time of the oophorectomy. By contrast, only 43% of the age-matched referent women had one or more condition. Therefore, it is possible that the causal mechanisms varied across women in the population (etiologic heterogeneity). Some women who had one or more chronic conditions at the time of the bilateral oophorectomy may have had some degree of accelerated aging preceding the surgery. Nevertheless, the oophorectomy caused hormonal disruption and an additional acceleration of the aging processes. In other women who did not have any of the 18 conditions at the time of the surgery, the oophorectomy initiated an abnormal pace of aging changes. Therefore, both the mechanism in FIGURE 3B and the mechanism in FIGURE 3C may be important in understanding the effects of sex steroids on the aging processes.
Possible Effect of Hormone Therapy
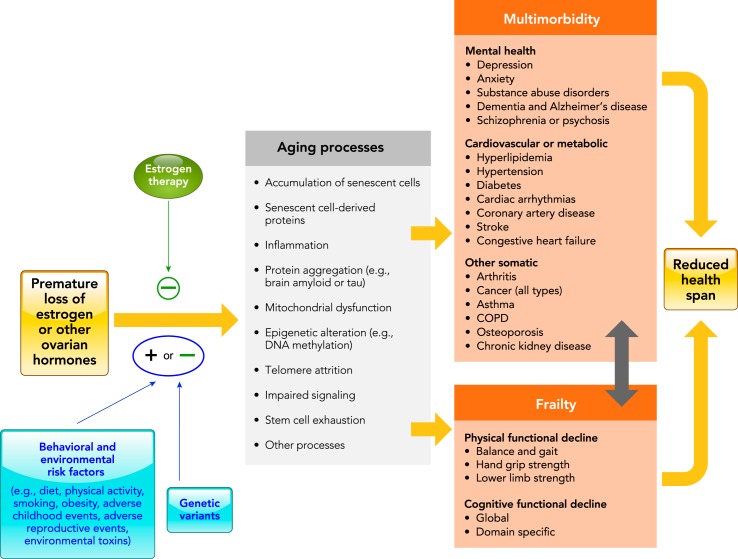
Women who underwent bilateral oophorectomy before age 46 yr and received ET to age 46 yr experienced a significant attenuation of the risk for osteoporosis compared with women who did not receive ET. However, we did not detect a significant interaction by ET for other outcomes such as hyperlipidemia or diabetes. Finally, we did not find a significant interaction with ET when we examined the effects of bilateral oophorectomy on the accumulation of multimorbidity. The failure to show significant interactions by ET may be due to the insufficient length of follow-up, to the limited statistical power, to the variable effect of ET across the different chronic conditions, or to the varying doses and formulations of ET used in past decades. We are continuing to follow the MOA-2 cohorts over time to study the effect of ET to age 50 yr (approximate age of natural menopause). The possible attenuating role of ET in the chain of causality linking bilateral oophorectomy with the mechanisms proposed to accelerate the aging processes needs rigorous investigation (FIGURE 4). In addition, it is important to consider that estrogen may exert protective or harmful effects on a given tissue depending on several different factors, including the timing of initiation of treatment, dose, mode of delivery, and formulation.
FIGURE 4.
Conceptual model of the possible relationship between bilateral oophorectomy, premature loss of estrogen, and accelerated aging
The central part of the figure was derived from figures in Refs. 10 and 33. Although in our model we postulate that estrogen plays a major role, the link between bilateral oophorectomy and accelerated aging may be mediated completely or in part by the loss of other ovarian hormones (e.g., premature loss of progesterone, testosterone, or inhibin, or disruption of the hypothalamus-pituitary-ovarian axis). Hormone therapy, primarily estrogen therapy, can attenuate the harmful effects of the premature loss of ovarian hormones caused by the oophorectomy. The minus sign indicates an antagonistic effect against accelerated aging (protective effect against aging). The genetic variants in the estrogen synthesis or responsiveness pathway may increase or decrease the effects of the hormonal loss (plus and minus sign). Similarly, behavioral or environmental risk factors may increase or decrease the effects of the hormonal loss (plus and minus sign). In this scenario, hormonal therapy, genetic factors, and non-genetic factors play a role as effect modifiers (interaction variables). COPD, chronic obstructive pulmonary disease.
Biomarkers of Aging
In 2016, the year of publication of the first MOA-2 paper, another group of investigators confirmed our findings of an association between bilateral oophorectomy and the pace of aging using a different approach. Instead of relating bilateral oophorectomy to the accelerated accumulation of multimorbidity, they examined the effects of bilateral oophorectomy on a biological marker of aging in blood, saliva, and buccal epithelium. The investigators assembled a group of 237 women included in the Women’s Health Initiative (WHI) study or in the Parkinson’s Disease, Environment, and Genes (PEG) study who underwent bilateral oophorectomy before the age of 50 yr (21). There was a significant association between bilateral oophorectomy and accelerated aging measured using a defined set of DNA methylation levels (single weighted average methylation level across multiple genomic regions) (16). Because DNA methylation is one of the best-known epigenetic mechanisms, the authors called their defined measure of methylation an “epigenetic clock” (16). Using this biomarker, age acceleration effects can be estimated by contrasting the age indicated by DNA methylation with the chronological age of a woman. Thus a woman whose blood has a higher DNA methylation age than expected based on her chronological age can be considered to have aged faster than expected (16).
The association with the methylation biomarker in blood was consistent across white women, black women, and Hispanic women from the WHI study, and in a group of primarily white women from the PEG study. In the PEG study, the association was also confirmed using the DNA biomarker derived from saliva but not using buccal epithelium (21). In addition, the authors reported a robust correlation between the biomarker in blood and in saliva, but not between the biomarker in blood and in buccal epithelium. Therefore, it is possible that different tissues undergo aging changes at a different pace in the same woman and that some specific risk or protective factors for aging (e.g., obesity) may be tissue-, organ-, or system-specific (17). On the other hand, it is also possible that the methylation measurement that was used does not perform homogeneously across tissues (technical limitation rather than biological variation). Levine and colleagues have recently refined their methylation biomarker measurement from blood to capture the risk for several diverse outcomes across multiple tissues and cells (22). However, this refined measurement has not been tested in women who underwent bilateral oophorectomy.
In support of a more general causal link between estrogen changes and accelerated aging, the authors also reported an association between early age of menopause and longer time since menopause with increased biological age as measured by the level of methylation in blood DNA. By contrast, they found reduced biological age associated with menopausal hormone therapy in buccal epithelium DNA (21). Although further work is needed, Levine and colleagues concluded that the premature loss of ovarian function may lead to an increase in epigenetic age, a biological marker of accelerated aging (16, 21).
We are aware of one additional study investigating the link between bilateral oophorectomy and other blood biomarkers related to aging. A 2014 Japanese study showed an association between bilateral oophorectomy and circulating levels of cytokines and chemokines. The levels of serum monocyte chemoattractant protein-1 (MCP-1) showed a significant increase (within 5 years after the surgery), whereas the level of serum interleukin (IL)-7 showed a significant decrease (≥5 years after the surgery) (45).
Our team (J.L.K., N.K.L., and M.J.S.) has focused recently on the accumulation of senescent cells and their possible role in causing inflammation and fibrosis. Senescent cells influence aging via the production of a senescence-associated secretory phenotype (SASP) (27, 39, 40). Additional studies are needed to test whether bilateral oophorectomy leads to a premature accumulation of senescent cells and to higher levels of SASP proteins in the blood, both in experimental animals and in women.
Cause and Effect Controversy
The two publications by Levine et al. and Rocca et al. provide important new evidence for a possible causal role of ovarian function in regulating the overall aging processes in women (21, 33). However, it remains difficult in a non-experimental setting to test definitively whether accelerated aging leads to symptoms that prompt bilateral oophorectomy or whether bilateral oophorectomy prompts hormonal changes leading to acceleration of aging processes (cause-effect uncertainty). In 2017, we addressed the cause-effect uncertainty with new analyses of the MOA-2 study restricted to women who did not have any of 18 chronic conditions at baseline (34). The MOA-2 cohorts were restricted to 420 premenopausal women who underwent bilateral oophorectomy before age 46 yr for a benign indication from 1988 to 2007 in Olmsted County, MN and age-matched referent women (±1 yr) who had not undergone bilateral oophorectomy. After adjustments for several possible confounding variables present at baseline, women who were free of any of the 18 chronic conditions and underwent bilateral oophorectomy experienced an accelerated rate of accumulation of the 18 chronic conditions considered together as a multimorbidity score (HR = 1.24; 95% CI = 1.12–1.37; P < 0.001). The single-year incidence rate of new conditions was most different between women with and without bilateral oophorectomy in the first 6 years after oophorectomy, but the difference attenuated thereafter. Importantly, the findings did not vary by surgical indication for the oophorectomy (i.e., women with or without a benign ovarian condition such as a cyst or endometriosis) (34).
Among women who underwent bilateral oophorectomy before age 46 yr and did not have any of the 18 conditions, those who received ET to age 46 yr did not have a significantly lower accumulation of multimorbidity compared with women who did not receive ET. As discussed before, this non-significant difference across the strata may be due to the insufficient length of follow-up, to the limited statistical power, to the variable effects of ET across the 18 chronic conditions, and/or to the variable doses and formulations of ET used in the past decades. In summary, current biomarker data and clinical evidence suggests that bilateral oophorectomy is causally linked to the acceleration of aging processes as measured by multimorbidity at the clinical level or by DNA methylation at the tissue level (21, 33, 34).
Overall Mechanisms
FIGURE 4 shows our overall mechanistic hypothesis linking premature loss of ovarian hormones caused by oophorectomy to the accelerated accumulation of multimorbidity and to the development of frailty, two complementary clinical proxy measures for the acceleration of aging processes (9, 10, 12). Some conditions may be affected by hormonal loss more severely or earlier in life than others. However, there is no consensus about how many conditions or clusters of conditions are needed or what severity of conditions is required to define accelerated aging and to separate low or moderate acceleration of aging processes. The greater rate of accumulation of chronic conditions with younger age at oophorectomy suggests that the hypothesized protective effects of ovarian hormones may be age-dependent and may have a critical age window (timing hypothesis), as already suggested in the case of cardiovascular and neurological diseases (7, 13, 14, 19, 30, 35, 36). This figure is somewhat simplified because it does not include the possible additional contribution of preexisting aging processes to the development of gynecological conditions prompting the oophorectomy (effect-cause mechanism; FIGURE 3C). The focus of FIGURE 4 is on the direct effects of oophorectomy on aging processes and on the effects that can be prevented by changing gynecological practice toward ovarian conservation.
It remains unclear whether the loss of estrogen is the major mediating consequence of bilateral oophorectomy. Other hormonal changes and a disruption of the HPO axis may play an important role. Current evidence concerning the effect of ET after bilateral oophorectomy suggests that ET may attenuate harmful effects but does not eliminate them. It is also possible that the dose, route of administration, and duration of ET used in the past decades were insufficient to achieve adequate levels of circulating estradiol. Doses of estradiol higher than the doses used typically in current clinical practice may be needed to approximate premenopausal blood levels of estradiol; however, more specific data are lacking. Some authors have suggested using preparations equivalent to 100 µg of transdermal estradiol or higher; however, clinical trials have not been conducted to evaluate these doses (11). Finally, other ovarian hormones may interact with estrogen in protecting tissues and organs from the aging processes.
Clinical Significance
Understanding the effects of bilateral oophorectomy on fundamental aging processes across different cells, tissues, organs, and systems is important to guide the decision of women and their gynecologists concerning removal or conservation of the ovaries in the context of treatment for a benign uterine or ovarian condition (32). There is now strong evidence against the use of bilateral oophorectomy for the prevention of breast and ovarian cancer in the majority of women who are at average risk of these cancers. However, although several professional societies worldwide have issued guidelines that discourage the practice of prophylactic bilateral oophorectomy, the practice continues (32, 33).
Understanding these mechanisms is also important for advancing research on aging and for suggesting possible therapeutic targets. This research is part of a broader research agenda focusing on other endocrine systems and their effect on multimorbidity and aging (e.g., thyroid function, adrenal function).
Conclusions
Bilateral oophorectomy in women younger than 46 yr (or younger than 50 yr) is a unique condition causing the premature loss of ovarian hormones, primarily estrogen, which is a disruptive endocrine event. The abrupt loss of ovarian hormones causes an alteration of several fundamental aging processes at the cellular, tissue, organ, and system levels, leading to multimorbidity, frailty, and reduced survival. However, many questions remain unanswered. New studies in animals are needed to investigate possible additional mechanisms at the cellular, tissue, and organ level that cannot be explored directly in women. New studies are also needed in women from different populations. Because the MOA-2 study included mainly white women, new studies are needed to confirm our findings in other populations both in the U.S. (e.g., blacks and Hispanics) and internationally (33). Interestingly, the association of bilateral oophorectomy with increased DNA methylation has already been confirmed across multiple racial groups (whites, blacks, and Hispanics) (21).
Although the MOA-2 study suggests that the harmful effects of oophorectomy may be reduced but not eliminated in women treated with estrogen at the dose and route of administration that were typically used in the last 20–30 yr (oral preparations with relatively low doses), newer formulations, modes of delivery, and doses of estrogen products need to be tested. New studies in women are needed to confirm the association between bilateral oophorectomy and acceleration of aging processes using biomarkers of aging at the tissue level (other than DNA methylation), using imaging (e.g., brain imaging), and using functional measures. New studies are also needed to investigate the association of bilateral oophorectomy with frailty as measured by physical functional decline (e.g., balance, gait, hand grip strength, lower limb strength) and cognitive functional decline (global or domain-specific; FIGURE 4). Finally, new studies in women should investigate the association of bilateral oophorectomy with imaging biomarkers of Alzheimer’s disease and of cerebrovascular disease. The biological mechanisms of aging that can be studied in women who have undergone bilateral oophorectomy may inform the development of interventions that may also apply to the aging processes in men.
Acknowledgments
The authors were partly supported by National Institute on Aging Grants R01 AG-034676, R01 AG-052425, P50 AG-044170, U01 AG-006786, and P01 AG-004875 (to W.A.R.); P50 AG-044170 (to V.M.M.); R37 AG-013925 (to J.L.K.); and R56 AG-052958 and R01 AG-052964 (to N.K.L.). J.L.K. was also partly supported by the Ted Nash Long Life and Noaber Foundations.
S.S.F. reports consulting fees from Mithra Pharmaceuticals and Procter & Gamble. E.A.S. reports grants from the National Institutes of Health and consulting fees from AbbVie, Bayer, GlaxoSmithKline, Gynesonics, Astellas Pharmaceuticals, Welltwigs, Viteava Pharmaceuticals, and Allergan, outside the submitted work. In addition, E.A.S. has a patent: Methods and Compounds for Treatment of Abnormal Uterine Bleeding (US 6440445 issued to None). K.K. serves on the data safety monitoring board for Takeda Global Research & Development Center, Inc. She receives research funding from the NIH, Alzheimer’s Drug and Discovery Foundation, and Avid Radiopharmaceuticals, Eli Lilly. M.M.M. served as a consultant to Eli Lilly and Lysosomal Therapeutics, Inc. She receives research support from the National Institute on Aging (R01 AG-049704, P50 AG-044170, U01 AG-006786, RF1 AG-055151), Department of Defense (W81XWH-15-1), and unrestricted research grants from Biogen and Lundbeck.
Author contributions: W.A.R. and V.M.M. conceived and organized the review; W.A.R. and C.Y.S. prepared the figures; W.A.R. drafted the manuscript; all authors reviewed and revised the manuscript; all authors approved the final version of the manuscript.
References
- 1.Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One 5: e10164, 2010. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedusi V, Meda C, Della Torre S, Monteleone G, Vegeto E, Maggi A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology 153: 2777–2788, 2012. doi: 10.1210/en.2011-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest 98: 36–42, 1996. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23: 665–686, 2002. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 5.Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci 268: 40–47, 2008. doi: 10.1016/j.jns.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Cid MC, Schnaper HW, Kleinman HK. Estrogens and the vascular endothelium. Ann N Y Acad Sci 966: 143–157, 2002. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson TB, Meléndez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause 20: 342–353, 2013. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 8.Dantas AP, Tostes RC, Fortes ZB, Costa SG, Nigro D, Carvalho MH. In vivo evidence for antioxidant potential of estrogen in microvessels of female spontaneously hypertensive rats. Hypertension 39: 405–411, 2002. doi: 10.1161/hy0202.102993. [DOI] [PubMed] [Google Scholar]
- 9.Fabbri E, An Y, Zoli M, Tanaka T, Simonsick EM, Kitner-Triolo MH, Studenski SA, Resnick SM, Ferrucci L. Association between accelerated multimorbidity and age-related cognitive decline in older Baltimore longitudinal study of aging participants without dementia. J Am Geriatr Soc 64: 965–972, 2016. doi: 10.1111/jgs.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc 16: 640–647, 2015. doi: 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faubion SS, Files JA, Rocca WA. When lowest dose for shortest amount of time does not apply. J Womens Health (Larchmt) 25: 416–417, 2016. doi: 10.1089/jwh.2016.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrucci L, Studenski SA. Clinical problems of aging. In: Harrison’s Principles of Internal Medicine (18th ed.), edited by Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J. New York, NY: McGraw-Hills, 2012. [Google Scholar]
- 13.Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev 31: 224–253, 2010. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev 95: 785–807, 2015. doi: 10.1152/physrev.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 15: 234–249, 2010. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 14: R115, 2013. doi: 10.1186/gb-2013-14-10-r115. An erratum to this article has been published in Genome Biol 16: 96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schönfels W, Ahrens M, Heits N, Bell JT, Tsai PC, Spector TD, Deloukas P, Siebert R, Sipos B, Becker T, Röcken C, Schafmayer C, Hampe J. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA 111: 15538–15543, 2014. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 29: 557–568, 2015. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JR, Manuck SB. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause 15: 768–776, 2008. doi: 10.1097/gme.0b013e31815eb18e. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima Y, Ono Y. Estrogens maintain skeletal muscle and satellite cell functions. J Endocrinol 229: 267–275, 2016. doi: 10.1530/JOE-15-0476. [DOI] [PubMed] [Google Scholar]
- 21.Levine ME, Lu AT, Chen BH, Hernandez DG, Singleton AB, Ferrucci L, Bandinelli S, Salfati E, Manson JE, Quach A, Kusters CD, Kuh D, Wong A, Teschendorff AE, Widschwendter M, Ritz BR, Absher D, Assimes TL, Horvath S. Menopause accelerates biological aging. Proc Natl Acad Sci USA 113: 9327–9332, 2016. doi: 10.1073/pnas.1604558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 10: 573–591, 2018. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav 66: 602–618, 2014. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci 64: 1207–1211, 2009. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci USA 96: 8867–8872, 1999. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The effects of oestrogens and their receptors on cardiometabolic health. Nat Rev Endocrinol 13: 352–364, 2017. doi: 10.1038/nrendo.2017.12. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15: 482–496, 2014. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 28.Porta M. (editor). A Dictionary of Epidemiology. New York: Oxford University Press, 2014. doi: 10.1093/acref/9780199976720.001.0001. [DOI] [Google Scholar]
- 29.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7: 715–726, 2011. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr, Roger VL, Melton LJ III, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 16: 15–23, 2009. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ III. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69: 1074–1083, 2007. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 32.Rocca WA, Faubion SS, Stewart EA, Miller VM. Salpingo-oophorectomy at the time of benign hysterectomy: a systematic review [letter]. Obstet Gynecol 129: 202–203, 2017. doi: 10.1097/AOG.0000000000001828. [DOI] [PubMed] [Google Scholar]
- 33.Rocca WA, Gazzuola-Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc 91: 1577–1589, 2016. doi: 10.1016/j.mayocp.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocca WA, Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM. Bilateral oophorectomy and accelerated aging: cause or effect? J Gerontol A Biol Sci Med Sci 72: 1213–1217, 2017. doi: 10.1093/gerona/glx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 7: 821–828, 2006. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 36.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol 389: 7–12, 2014. doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ III. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond) 5: 39–48, 2009. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med 26: 20–36, 2007. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 39.Schafer MJ, Miller JD, LeBrasseur NK. Cellular senescence: implications for metabolic disease. Mol Cell Endocrinol 455: 93–102, 2017. doi: 10.1016/j.mce.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532, 2017. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev 24: 133–151, 2003. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 42.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86: 225–230, 2003. doi: 10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 43.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab 83: 1875–1880, 1998. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 44.St Sauver JL, Boyd CM, Grossardt BR, Bobo WV, Finney Rutten LJ, Roger VL, Ebbert JO, Therneau TM, Yawn BP, Rocca WA. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open 5: e006413, 2015. doi: 10.1136/bmjopen-2014-006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tani A, Yasui T, Matsui S, Kato T, Tsuchiya N, Yuzurihara M, Kase Y, Irahara M. Circulating levels of monocyte chemoattractant protein-1 and interleukin-7 in women who have undergone bilateral salpingo-oophorectomy. J Inflamm Res 7: 1–7, 2013. doi: 10.2147/JIR.S52728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR, Neufer PD. 17Beta-estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab 27: 167–179.e7, 2018. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vetrano DL, Calderón-Larrañaga A, Marengoni A, Onder G, Bauer JM, Cesari M, Ferrucci L, Fratiglioni L. An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol A Biol Sci Med Sci. In press. doi: 10.1093/gerona/glx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J. The many faces of estrogen signaling. Biochem Med (Zagreb) 24: 329–342, 2014. doi: 10.11613/BM.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waters DJ, Kengeri SS, Clever B, Booth JA, Maras AH, Schlittler DL, Hayek MG. Exploring mechanisms of sex differences in longevity: lifetime ovary exposure and exceptional longevity in dogs. Aging Cell 8: 752–755, 2009. doi: 10.1111/j.1474-9726.2009.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weel AE, Uitterlinden AG, Westendorp IC, Burger H, Schuit SC, Hofman A, Helmerhorst TJ, van Leeuwen JP, Pols HA. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J Clin Endocrinol Metab 84: 3146–3150, 1999. doi: 10.1210/jcem.84.9.5981. [DOI] [PubMed] [Google Scholar]
- 51.Wilson LF, Pandeya N, Byles J, Mishra GD. Hysterectomy and perceived physical function in middle-aged Australian women: a 20-year population-based prospective cohort study. Qual Life Res 27: 1501–1511, 2018. doi: 10.1007/s11136-018-1812-9. [DOI] [PubMed] [Google Scholar]