Highlights
-
•
Proton pelvic reirradiation results in lower bone marrow dose compared to photons.
-
•
Proton pelvic reirradiation of the pelvis is well tolerated with limited toxicities.
Keywords: Proton re-irradiation, Rectal cancer, Anal cancer, Recurrent cancer, Proton beam radiation
Abstract
Introduction
Pelvic reirradiation (re-RT) presents challenges due to concerns for late toxicity to tissues-at-risk including pelvic bone marrow (PBM). We routinely utilize a hyperfractionated, accelerated re-RT for recurrent rectal or anal cancer in the setting of prior radiation. We hypothesized that proton beam radiation (PBR) is uniquely suited to limit doses to pelvic non-target tissues better than photon-based approaches.
Materials and methods
All patients who received hyperfractionated, accelerated PBR re-RT to the pelvis from 2007 to 2017 were identified. Re-RT was delivered twice daily with a 6 h minimum interfraction interval at 1.5 Gray Relative Biological Effectiveness (Gy(RBE)) per fraction to a total dose of 39–45 Gy(RBE). Concurrent chemotherapy was given to all patients. Comparison photon plans were generated for dosimetric analysis. Dosimetric parameters compared using a matched-pair analysis and the Wilcoxon signed-rank test. Survival analysis was performed Kaplan Meier curves.
Results
Fifteen patients were identified, with a median prior pelvic RT dose of 50.4 Gy (range 25–80 Gy). Median time between the initial RT and PBRT re-RT was 4.7 years (range 1.0–36.1 years). In comparison to corresponding photon re-RT plans, PBR re-RT plans had lower mean PBM dose, and lower volume of PBM getting 5 Gy, 10 Gy, 20 Gy, and 30 Gy (p < 0.001, p < 0.001, p < 0.001, and p = 0.033, respectively).
With median 13.9 months follow-up after PBR re-RT, five patients had developed local recurrences, and four patients had developed distant metastases. One-year overall survival following PBR re-RT was 67.5% and one-year progression free survival was 58.7%. No patients developed acute or late Grade 4 toxicity.
Conclusion
PBR re-RT affords improved sparing of PBM compared with photon-based re-RT. Clinically, PBR re-RT is well-tolerated. However, given modest control rates with definitive re-RT without subsequent surgical resection, a multidisciplinary approach should be favored in this setting when feasible.
1. Introduction
Lower gastrointestinal (GI) cancers arising in a previously irradiated pelvic field can prove challenging to treat. This situation can occur either when a previously irradiated tumor recurs locally or when a new primary tumor arises in an area treated with radiation therapy (RT) for a prior malignancy. The best curative treatment option is typically surgical resection. However, delayed surgery after radiation leads to increased risk of intraoperative and post-operative morbidity due to radiation-induced fibrosis [1].
Historically, there has been a reluctance to offer pelvic reirradiation due to concerns of the cumulative dose to adjacent organs-at-risk (OARs) leading to unacceptable toxicities. Radiosensitive luminal GI organs such as the small and large intestines are particularly at risk for late toxicities such as ulceration, bleeding, perforation and fistula if doses exceed reported tolerance levels [2], [3]. More recently, however, several retrospective and prospective studies have reported encouraging outcomes of pelvic reirriadiation for recurrent rectal adenocarcinoma [4], [5], [6], [7], [8], [9], [10], [11]. Other studies show feasibility of reirradiation in the treatment of anal or rectal cancer after having received pelvic RT for a different malignancy [12], [13]. Most published studies have utilized an accelerated hyperfractionated schedule of 1.2–1.5 Gray (Gy) per fraction delivered twice daily to a total dose of 30–45 Gy, as standard fractionation in the reirradiation setting has been associated with higher late toxicity rates [14].
Even though reirradiation using an accelerated, hyperfractionated schedule appears to be safe and effective, as many as one third of patients develop grade 3–4 late toxicities including small bowel obstruction, ureteral stricture, urinary obstruction, anastomotic leak or stricture, chronic diarrhea and fistula formation [5]. As such, more advanced and focal radiation technologies have been studied in an effort to reduce toxicity rates and improve the therapeutic ratio [15], [16], [17], [18], [19], [20], [21], [22]. Particle therapy allows for more conformal radiation delivery by minimizing unnecessary exit dose beyond the tumor target. Carbon ion therapy has been utilized in this setting, and a prospective trial is currently underway [23], [24]. Proton beam radiation (PBR) similarly has the potential to reduce dose to OARs in the pelvis, including the pelvic bone marrow, which may lead to decreased hematologic and non-hematologic toxicity [25].
The aims of this study were (1) to describe toxicity and local control outcomes for patients treated with definitive accelerated, hyperfractionated PBR in the reirradiation setting and (2) to compare doses to the bowel, bladder, femoral heads and pelvic bone marrow between proton and photon reirradiation plans.
2. Materials and methods
This study was approved by the institutional review board. Patients who underwent reirradiation with accelerated, hyperfractionated PBR therapy from 2007 to 2017 were identified from institutional databases and included for analysis. Medical records were accessed retrospectively and patient data were collected and analyzed.
2.1. Radiation therapy
All patients were discussed in the multidisciplinary setting before starting treatment. All included patients were not considered candidates for curative resection. After being dispositioned to definitive PBR re-RT, patients underwent a computed tomography (CT) simulation for radiation treatment planning. They were positioned supine with a full bladder, if appropriate, to displace small bowel out of the pelvic treatment field(s). Proton plans were generated using a passive scatter technique with 2–3 beams. Bladder, pelvic bone marrow (PBM) and bowel bag were contoured as described previously [26], [27]. When available, composite plans were generated with the prior RT plan. However, some patients were treated >10 years prior or at outside institutions, which made it impossible to generate composite plans for all patients. No evidenced-based dose constraints exist for the reirradiation setting. In treatment planning for PBR re-RT, efforts were made to decrease hot spots in bowel, particularly if the bowel received full dose in the first course of radiation. Comparison photon plans were generated for dosimetric analysis using a 3D conformal technique [5].
Patients received reirradiation twice daily with a 6 h minimum inter-fraction interval. PBR was given at 1.5 Gy (RBE) per fraction to a total dose of 39–45 Gy (RBE). Concurrent chemotherapy was given to all patients; 5-fluorouracil (5FU) or capecitabine for those with rectal adenocarcinoma and 5FU plus cisplatin for those with anal squamous cell carcinoma.
2.2. Toxicity
Acute (occurring within 90 days of radiation therapy) and late (occurring after 90 days post-RT) toxicity outcomes for patients were determined using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 scale during retrospective chart review. Complete blood count with differential was obtained at least weekly during PBR treatment and again at follow up 6–12 weeks after completion of reirradiation.
2.3. Statistical analysis
Disease-related outcomes analyzed included overall survival (OS) and progression-free survival (PFS); both were calculated from initiation of PBR re-RT. Survival curves were generated using the Kaplan-Meier method. Dosimetric comparisons were performed matched-pair analysis with Wilcoxon signed-rank testing. Analyses were performed using SPSS (Version 22.0, Armonk, NY).
3. Results
Fifteen patients were identified as meeting inclusion criteria for analysis. The median (range) age was 74 (55–91) years, 8 patients (53.3%) were male. and the median follow up was 13.9 months (range, 1–37.9 months). All patients had a history of definitive or preoperative RT to the pelvis; eight patients were initially treated with 3D conformal radiation therapy (3DCRT) and 7 patients with IMRT for their initial disease to a median (range) dose of 50.4 Gy (25–80 Gy). Median (range) time between the first and second course of radiation therapy was 4.7 (0.99–36.1) years. Further tumor and treatment details are outlined in Table 1.
Table 1.
Patient Characteristics.
| Initial Histology | Current Histology | Initial RT dose (Gy) | Initial # fractions | Re-RT dose (Gy(RBE)) | Re-RT interval (years) | Acute toxicity | Late toxicity |
|---|---|---|---|---|---|---|---|
| RAC* | Adeno ⱡⱡ | 25 | 5 | 36 | 0.9 | None | None |
| RAC* | Adeno ⱡⱡ | 54 | 27 | 36 | 25.7 | G1 diarrhea | None |
| RAC* | Adeno ⱡⱡ | 50.4 | 28 | 39 | 10.7 | None | None |
| RAC* | Adeno ⱡⱡ | 50.4 | 28 | 42 | 5.5 | G1 pain, G3 lymphopenia | None |
| RAC* | Adeno ⱡⱡ | 50.4 | 28 | 39 | 18.8 | G1 pain, dermatitis and constipation | None |
| RAC* | Adeno ⱡⱡ | 45 | 25 | 39 | 1.5 | G1 diarrhea and fatigue | None |
| ASCC** | SCCⱡ | 50.4 | 28 | 45 | 2.2 | None | None |
| ASCC** | SCCⱡ | 54 | 30 | 45 | 3.3 | G1 diarrhea, fatigue, nausea, pain and G2 dermatitis | None |
| ASCC** | SCCⱡ | 50 | 25 | 45 | 4.7 | G1 fatigue, nausea, constipation, dermatitis | G2 pelvic fracture |
| ASCC** | SCCⱡ | 54 | 30 | 39 | 2.4 | G1 nausea, pain, diarrhea, fatigue | None |
| ASCC** | SCCⱡ | 50.4 | 28 | 39 | 1.4 | G1 nausea, fatigue | G3 dysuria, G1 pain |
| PAC*** | Adeno ⱡⱡ | 75.6 | 42 | 45 | 7.7 | G1 diarrhea, fatigue, hematochezia | None |
| PAC*** | Adeno ⱡⱡ | 80 | 40 | 45.5 | 14.1 | G2 diarrhea, mucositis, pain | G3 rectal bleeding |
| Lymphoma | Adeno ⱡⱡ | 40 | 20 | 29 | 36.1 | G1 pain, fatigue, diarrhea, headache | None |
| Neuro-endocrine carcinoma | Neuro-endocrine carcinoma | 54 | 27 | 39 | 1.6 | G1 fatigue, dysuria and G2 pain | None |
*RAC: Rectal Adenocarcinoma; ** ASCC: Anal Squamous Cell Carcinoma; ***PAC: Prostate Adenocarcinoma; ⱡSCC: Squamous Cell Carcinoma; ⱡⱡ Adeno: Adenocarcinoma
3.1. Dosimetric comparison
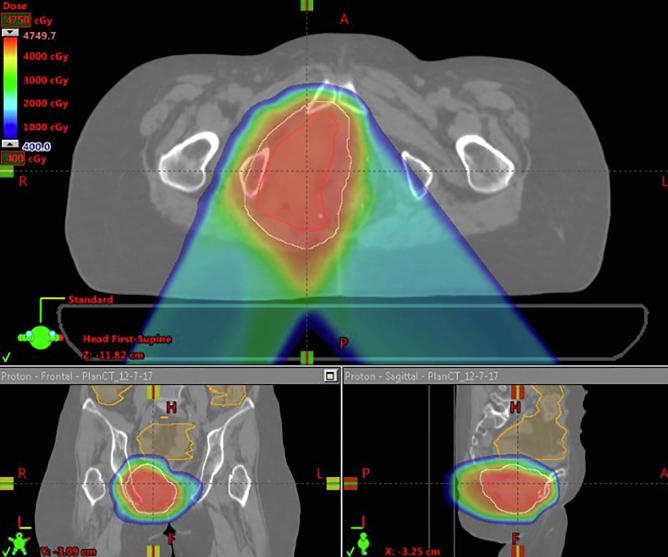
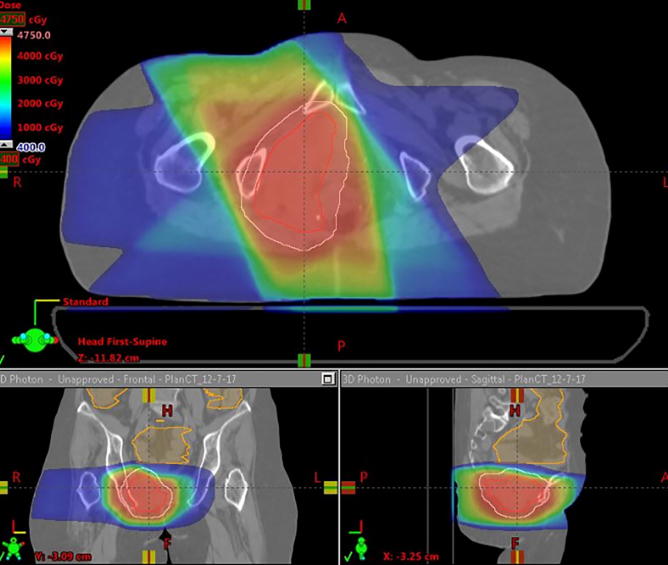
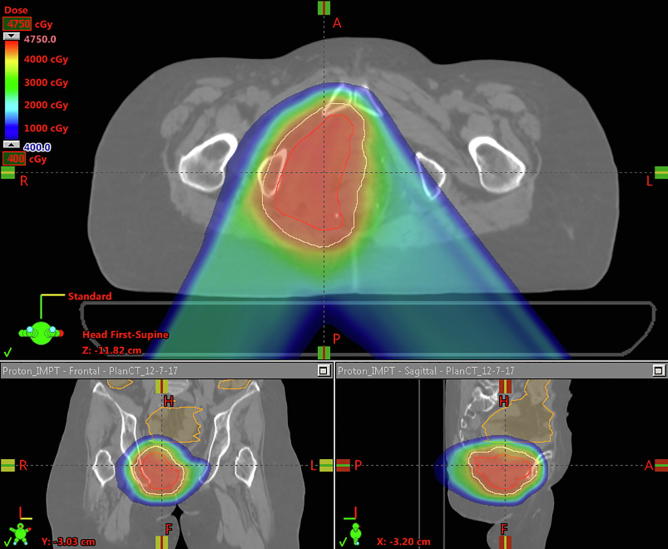
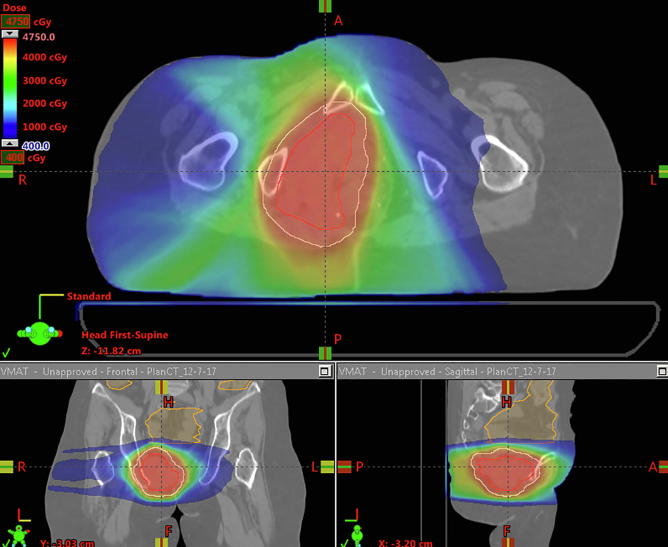
All patients were treated with PBR but had comparison photon plans generated, and doses to organs at risk (OARs) were compared. PBR plans had a lower mean pelvic bone marrow (PBM) dose, lower volume of PBM getting 5 Gy (V5), 10 Gy (V10), 20 Gy (V20) and 30 Gy (V30) (p < 0.001, p < 0.001, p < 0.001 and p = 0.033 respectively). Additionally PBR plans had lower volumes of bowel getting 30 Gy or more (V30) (p = 0.017). There were no significant differences between PBR and photon plans with regard to maximum bowel or bladder dose (Table 2). Fig. 1A, Fig. 1B, Fig. 1C, Fig. 1D highlights comparisons between passive protons (Fig. 1A), 3D conformal (Fig. 1B), Intensity modulated proton therapy (Fig. 1C) and Volumetric Arc Therapy (Fig. 1D) plans.
Table 2.
Dosimetric Parameters.
| Dosimetric Parameter | Proton (Mean ± SE) | Photon (Mean ± SE) | P-value |
|---|---|---|---|
| Bowel Max Dose | 36.2 ± 2.5 Gy(RBE) | 32.4 ± 3.8 Gy | 0.20 |
| Bowel V30 | 3.9 ± 2.0% | 16.1 ± 6.2% | 0.017* |
| Bladder Max Dose | 33.4 ± 4.1 Gy(RBE) | 36.1 ± 3.3 Gy | 0.30 |
| Bladder V30 | 14.6 ± 4.4% | 23.3 ± 6.3% | 0.050 |
| Left Femur Max | 17.3 ± 4.5 Gy (RBE) | 26.2 ± 3.4 Gy | 0.018* |
| Right Femur Max | 15.7 ± 3.4 Gy (RBE) | 28.0 ± 3.5 Gy | 0.001* |
| Pelvic Bone Marrow Mean | 5.8 ± 1.3 Gy(RBE) | 11.1 ± 2.1 Gy | <0.001* |
| Pelvic Bone Marrow V5 | 21.3 ± 4.4% | 58.1 ± 7.2% | <0.001* |
| Pelvic Bone Marrow V10 | 18.6 ± 4.0% | 46.7 ± 7.7% | <0.001* |
| Pelvic Bone Marrow V20 | 13.1 ± 3.4% | 27.2 ± 6.3% | <0.001* |
| Pelvic Bone Marrow V30 | 9.7 ± 2.6% | 12.1 ± 3.4% | 0.033 |
* Statistically Significant p-value (P < 0.05).
Fig. 1A.
Proton Plan for a patient receiving with recurrent squamous cell carcinoma of the anus who received proton re-irradiation to 45 Gy(RBE) in 30 fractions.
Fig. 1B.
A Photon plan planned to 45 Gy in 30 fractions for the same patient as above.
Fig. 1C.
IMPT plan to 45 Gy in 30 fractions for the same patient as above.
Fig. 1D.
VMAT plan to 45 in 30 fractions for the same patient as above.
3.2. Oncologic outcomes
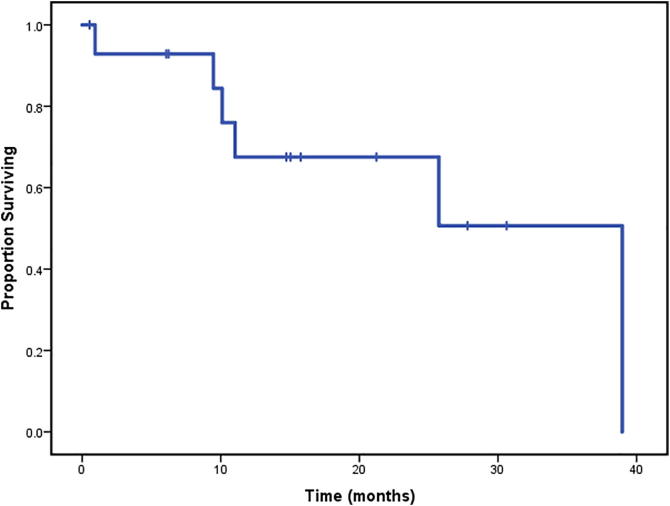
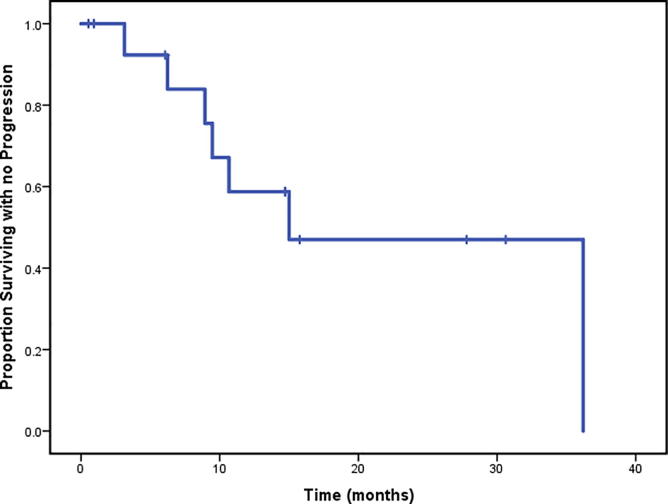
With a median 13.9 months follow-up after completion of PBR re-RT, a total of three patients had developed local recurrences only, two developed both local recurrence and distant metastases and two patients had developed distant metastases only. All four patients who developed distant metastases died. One patient who developed a local recurrence died of unknown causes and a second patient without a local recurrence died of an aggressive acute leukemia approximately 9 months after completion of PBR reirradiation. Median OS from start of re-RT was 39.0 months (range, 0.5–38.9 months). 1-year and 2-year OS rates were 67.5% and 67.5% respectively (Fig. 2A). Median PFS was 15.0 months (range, 0.5–36.2 months) from start of re-RT. 1-year and 2-year PFS rates were 58.7% and 47% respectively (Fig. 2B).
Fig. 2A.
Overall survival of the entire cohort (N = 15).
Fig. 2B.
Progression free survival of the entire cohort (N = 15).
3.3. Toxicity
Ten patients developed grade 1–2 acute toxicities. Grade 1 toxicities included diarrhea, fatigue, hematochezia, nausea, pain, constipation and dermatitis. Grade 2 toxicity included diarrhea, mucositis and pain. With the exception of one patient with Grade 3 lymphopenia, there were no additional grade 3 and no grade 4 toxicities. All patients completed treatment as prescribed without the need for treatment breaks. Three patients developed late toxicity six or more weeks after radiation; one patient developed grade 1 pain and grade 3 dysuria. A second patient developed grade 3 rectal bleeding. Finally, a third patient developed a grade 2 pelvic fracture.
4. Discussion
Our study is the largest cohort of patients receiving definitive reirradiation with accelerated, hyperfractionated PBR for treatment of lower GI malignancies arising in a previously irradiated field to date. These patients tolerated accelerated, hyperfractionated PBR well with minimal acute and late RT-related toxicities. Additionally, in our dosimetric comparison, we found that re-irradiation with PBR leads to significantly lower volumes of pelvic bone marrow getting 5, 10, 20 and 30 Gy of radiation.
The management of lower GI tumors arising in a previously irradiated field continues to represent a clinical challenge. The efficacy and tolerability of 30–40 Gy in an accelerated, hyperfractionated regimen with photon-based techniques has been demonstrated, though with grade 3–4 toxicity occurring an approximately one third of patients [5]. In efforts to increase dose to the tumor while minimizing dose to OARs, several advanced techniques have been tried with varying degrees of success. Stereotactic body radiotherapy (SBRT) has been utilized to salvage gynecologic cancer and prostate cancer recurrences after prior radiation, but a meta-analysis of gynecologic cancer reirradiation with SBRT reported a grade 3–4 toxicity rate of approximately 20% [15], [16]. Fewer studies have reported results of SBRT in the reirradiation setting for lower GI cancers, and most reported treatments have been in the setting of pre-sacral or nodal recurrences, not anastamotic or luminal recurrences [17], [18]. Brachytherapy is another focal radiation modality frequently used for reirradiation of gynecologic and prostate malignancies [19], [20]. Small series of high dose-rate endorectal and or interstitial brachytherapy have been published looking at treatment in the preoperative setting for the treatment of recurrent rectal cancers [21], [22].
PBR shows promise in its ability to minimize dose to non-target tissues given the physical properties of protons. Berman et al. prospectively investigated PBR for seven patients with locally recurrent rectal cancer [25]. Their reirradiation dose was slightly higher than our cohort at a mean of 61.20 Gy (RBE) with a range from 45 to 64.80 Gy delivered using once daily standard fractionation. With 39 months of follow up, four of seven patients had a complete metabolic response. However, Berman et al reported higher moderate to severe toxicity rates compared to our cohort, with three patients experiencing acute grade 3 toxicities and three patients have late grade 4 toxicities. The reported acute grade 3 toxicities included abdominal pain and diarrhea and late grade 4 toxicities included two patients with bowel obstruction and one who developed an entero-vaginal fistula [25]. Our toxicity rate was lower likely because of the unique combination of PBR with the accelerated, hyperfractionated schedule.
Results of PBR in the reirradiation setting have been published primarily for upper abdominal cancers, particularly hepatobiliary malignancies. However, most have used either standard daily fractionation or a hypofractionated regimen. Boimel et al. evaluated 15 patients with recurrent pancreatic cancer who were treated with PBR for their recurrence and had a 16 month follow up [28]. The median re-irradiation dose was 59.4 Gy (RBE) (range: 37.5–59.4 Gy). The reported toxicity rate was also higher than in our cohort with acute grade 3 toxicities (anorexia and fatigue) one grade 4 duodenal ulcer and a grade 5 bowel perforation. Another reirradiation proton study from Japan examined patients with hepatocellular carcinoma getting reirradiation with PBR with initial RT also having been delivered with PBR [29]. The most common doses for re-irradiation in this cohort of 83 patients was 66, 72.6 and 74 Gy (RBE) in 10, 22 and 37 fractions respectively. Acute and late toxicity rates were very low, though minimal bowel was subjected to reirradiation [29]. With pelvic GI tumor target volumes close to previously irradiated bowel and bladder, we believe that the unique combination of the accelerated, hyperfractionated schedule along with PBR has the potential to further minimize toxicity.
Some limitations to our study include the small sample size, single institution experience, and retrospective study design. However, even with these limitations, this study provides a substantial contribution to the currently-scant literature on definitive PBR reirradiation for lower GI malignancies within the pelvis. Here, we demonstrate dosimetric advantages of using PBR versus photon-based re-RT; our series highlights low toxicity rates when PBR re-RT is delivered using an accelerated, hyperfractionated schedule. However, the median progression-free survival was modest at best, so alternate means of safe dose escalation should be explored for previously irradiated patients with inoperable pelvic GI tumors.
5. Conclusions
PBR re-RT affords improved sparing of PBM compared with photon-based re-RT. Clinically, PBR re-RT is well-tolerated. However, given modest control rates with definitive re-RT without subsequent surgical resection, a multidisciplinary approach should be favored in this setting when feasible.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rosoff J.S., Savage S.J., Prasad S.M. Salvage radical prostatectomy as management of locally recurrent prostate cancer: outcomes and complications. World J Urol. 2013;31(6):1347–1352. doi: 10.1007/s00345-013-1029-z. [DOI] [PubMed] [Google Scholar]
- 2.Emami B., Lyman J., Brown A., Coia L., Goitein M., Munzenrider J.E. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas S., Chen L., Choflet A., Fader A., Guss Z., Hazell S. Pelvic radiation and normal tissue toxicity. Semin Radiat Oncol. 2017;27(4):358–369. doi: 10.1016/j.semradonc.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Owens R., Muirhead R. External beam re-irradiation in rectal cancer. Clin Oncol (R Coll Radiol) 2018;30(2):116–123. doi: 10.1016/j.clon.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Tao R., Tsai C.J., Jensen G., Eng C., Kopetz S., Overman M.J. Hyperfractionated accelerated reirradiation for rectal cancer: an analysis of outcomes and toxicity. Radiother Oncol. 2017;122(1):146–151. doi: 10.1016/j.radonc.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Das P., Delclos M.E., Skibber J.M., Rodriguez-Bigas M.A., Feig B.W., Chang G.J. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J. Radiat Oncol, Biol, Phys. 2010;77(1):60–65. doi: 10.1016/j.ijrobp.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 7.Susko M., Lee J., Salama J., Thomas S., Uronis H., Hsu D. The use of re-irradiation in locally recurrent, non-metastatic rectal cancer. Ann Surg Oncol. 2016;23(11):3609–3615. doi: 10.1245/s10434-016-5250-z. [DOI] [PubMed] [Google Scholar]
- 8.Kusters M., Bosman S.J., Van Zoggel D.M., Nieuwenhuijzen G.A., Creemers G.J., Van den Berg H.A. Local recurrence in the lateral lymph node compartment: improved outcomes with induction chemotherapy combined with multimodality treatment. Ann Surg Oncol. 2016;23(6):1883–1889. doi: 10.1245/s10434-016-5098-2. [DOI] [PubMed] [Google Scholar]
- 9.Mohiuddin M., Lingareddy V., Rakinic J., Marks G. Reirradiation for rectal cancer and surgical resection after ultra high doses. Int J Radiat Oncol Biol Phys. 1993;27(5):1159–1163. doi: 10.1016/0360-3016(93)90538-7. [DOI] [PubMed] [Google Scholar]
- 10.Valentini V., Morganti A.G., Gambacorta M.A., Mohiuddin M., Doglietto G.B., Coco C. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64(4):1129–1139. doi: 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Cai G., Zhu J., Hu W., Zhang Z. Accelerated hyperfractionated intensity-modulated radiotherapy for recurrent/unresectable rectal cancer in patients with previous pelvic irradiation: results of a phase II study. Radiat Oncol. 2014;9:278. doi: 10.1186/s13014-014-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborne E.M., Eng C., Skibber J.M., Rodriguez-Bigas M.A., Chang G.J., Nancy You Y.Q. Hyperfractionated accelerated reirradiation for patients with recurrent anal cancer previously treated with definitive chemoradiation. Am J Clin Oncol. 2018;41(7):632–637. doi: 10.1097/COC.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 13.Jensen G., Tao R., Eng C., Skibber J.M., Rodriguez-Bigas M., Chang G.J. Treatment of primary rectal adenocarcinoma after prior pelvic radiation: the role of hyperfractionated accelerated reirradiation. Adv Radiat Oncol. 2018;3(4):595–600. doi: 10.1016/j.adro.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohiuddin M., Marks G., Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95(5):1144–1150. doi: 10.1002/cncr.10799. [DOI] [PubMed] [Google Scholar]
- 15.Mendez L.C., Leung E., Cheung P., Barbera L. The role of stereotactic ablative body radiotherapy in gynaecological cancers: a systematic review. Clin Oncol (R Coll Radiol) 2017;29(6):378–384. doi: 10.1016/j.clon.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Jereczek-Fossa B.A., Rojas D.P., Zerini D., Fodor C., Viola A., Fanetti G. Reirradiation for isolated local recurrence of prostate cancer: mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT) Br J Radiol. 2019;92(1094):20180494. doi: 10.1259/bjr.20180494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defoe S.G., Bernard M.E., Rwigema J.C., Heron D.E., Ozhasoglu C., Burton S. Stereotactic body radiotherapy for the treatment of presacral recurrences from rectal cancers. J Cancer Res Ther. 2011;7(4):408–411. doi: 10.4103/0973-1482.92000. [DOI] [PubMed] [Google Scholar]
- 18.Kim M.S., Choi C., Yoo S., Cho C., Seo Y., Ji Y. Stereotactic body radiation therapy in patients with pelvic recurrence from rectal carcinoma. Jpn J Clin Oncol. 2008;38(10):695–700. doi: 10.1093/jjco/hyn083. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Monge R., Cambeiro M., Rodriguez-Ruiz M.E., Olarte A., Ramos L.I., Villafranca E. Phase II trial of image-based high-dose-rate interstitial brachytherapy for previously irradiated gynecologic cancer. Brachytherapy. 2014;13(3):219–224. doi: 10.1016/j.brachy.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Quivrin M., Peignaux-Casasnovas K., Martin E., Rouffiac M., Thibouw D., Chevalier C. Salvage brachytherapy as a modern reirradiation technique for local cancer failure: the Phoenix is reborn from its ashes. CancerRadiother. 2018;22(4):372–381. doi: 10.1016/j.canrad.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Chuong M.D., Fernandez D.C., Shridhar R., Hoffe S.E., Saini A., Hunt D. High-dose-rate endorectal brachytherapy for locally advanced rectal cancer in previously irradiated patients. Brachytherapy. 2013;12(5):457–462. doi: 10.1016/j.brachy.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Pellizzon A.C. Salvage high-dose-rate interstitial brachytherapy for locally recurrent rectal cancer. Radiol Bras. 2016;49(3):196–198. doi: 10.1590/0100-3984.2013.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habermehl D., Wagner M., Ellerbrock M., Buchler M.W., Jakel O., Debus J. Reirradiation Using Carbon Ions in Patients with Locally Recurrent Rectal Cancer at HIT: First Results. Ann Surg Oncol. 2015;22(6):2068–2074. doi: 10.1245/s10434-014-4219-z. [DOI] [PubMed] [Google Scholar]
- 24.https://clinicaltrials.gov/ct2/show/NCT01528683.
- 25.Berman A.T., Both S., Sharkoski T., Goldrath K., Tochner Z., Apisarnthanarax S. Proton reirradiation of recurrent rectal cancer: dosimetric comparison, toxicities, and preliminary outcomes. Int J Particle Ther. 2014;1(1):2–13. [Google Scholar]
- 26.Mell L.K., Kochanski J.D., Roeske J.C., Haslam J.J., Mehta N., Yamada S.D. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(5):1356–1365. doi: 10.1016/j.ijrobp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Kavanagh B.D., Pan C.C., Dawson L.A., Das S.K., Li X.A., Ten Haken R.K. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S101–S107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 28.Boimel P.J., Berman A.T., Li J., Apisarnthanarax S., Both S., Lelionis K. Proton beam reirradiation for locally recurrent pancreatic adenocarcinoma. J Gastrointestinal Oncol. 2017;8(4):665. doi: 10.21037/jgo.2017.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshiro Y., Mizumoto M., Okumura T., Fukuda K., Fukumitsu N., Abei M. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother Oncol. 2017;123(2):240–245. doi: 10.1016/j.radonc.2017.03.004. [DOI] [PubMed] [Google Scholar]