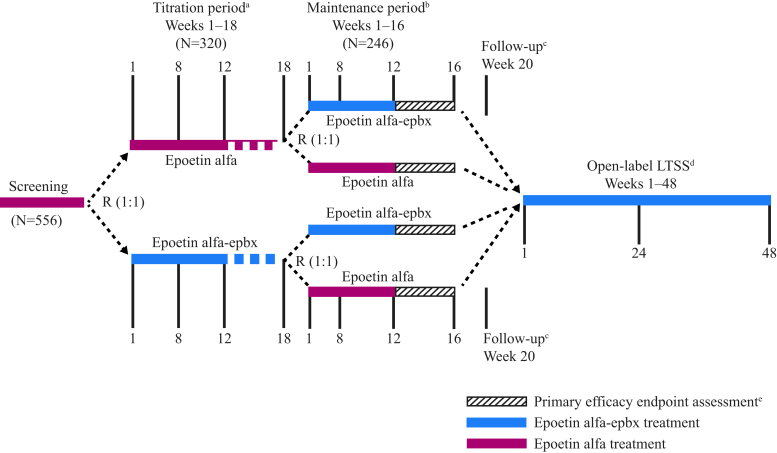
Figure 1.
Study design. aPatients who were on subcutaneous treatment at the time of screening and demonstrated optimal stable dosing for 4 consecutive weeks were randomized (R) into the titration phase, received study drug assignment, and then proceeded directly to the maintenance phase if they satisfied all other study criteria for entry into the maintenance phase. Patients who did not demonstrate optimal and stable dosing by week 18 did not continue to the maintenance phase. bPatients who discontinued the study drug early during the maintenance phase received standard-of-care erythropoiesis-stimulating agent treatment until either the follow-up visit or entry into the long-term safety study (LTSS). cPatients who did not enter the LTSS underwent a follow-up visit, 4 weeks after the last schedule of study activities. dPatients had up to 28 days from completion of week 16 of the maintenance phase to enter into the LTSS. ePrimary efficacy endpoints will be assessed during the last 4 weeks of the maintenance phase.