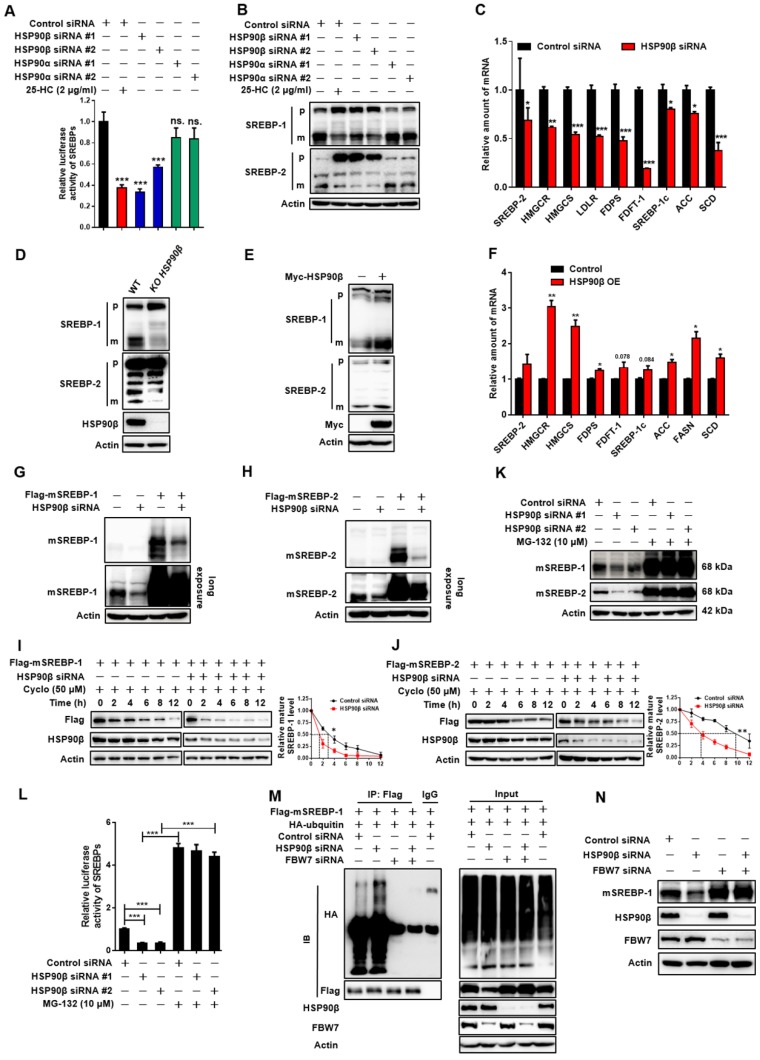
Figure 3.
HSP90β Regulates de novo Lipid Synthesis through Promoting the mSREBPs Ubiquitination and Proteasomal degradation. (A) HL-7702/SRE-Luc cells were incubated in medium B and treated with indicated siRNA for 48 h, the cells were then switched to medium D or medium D containing 25-HC (2 μg/ml) for 4 h. After the treatment, cells were lysed and luciferase activity was measured. (B and C) HL-7702 hepatocytes were incubated in medium B and treated with indicated siRNA for 48 h, the cells were then switched to medium D or medium D containing 25-HC (2 μg/ml) for 4 h. After the treatment, (B) the whole cell extracts underwent immunoblotting with indicated antibodies. P represents precursor SREBP and m represents mSREBP. (C) The expression of various genes was analyzed by qRT-PCR. Human GAPDH was used as the control. (D) The HL-7702/WT and HL-7702/KO HSP90β cells were depleted of sterols by incubating in medium D for 24 h, the total protein of cells were prepared and subjected to immunoblotting with indicated antibodies. (E and F) HL-7702 hepatocytes were transfected with myc-HSP90β (3.4 times) for 24 h, and then depleted of sterols by incubating in medium D for another 24 h, (E) the whole cell extracts underwent immunoblotting with indicated antibodies. (F) The expression of various genes was analyzed by qRT-PCR. Human GAPDH was used as the internal control. (G and H) HL-7702 hepatocytes were transfected with flag-mSREBP-1 (G) or flag-mSREBP-2 (H) plasmids and cultured for 24 h. The cells were switched to medium D for another 24 h, the whole cell extracts underwent immunoblotting with indicated antibodies. (I and J) HL-7702 hepatocytes were transfected with flag-mSREBP-1 plasmid and siRNA against HSP90β for 48 h (I). HL-7702 hepatocytes were transfected with flag-mSREBP-2 plasmid and siRNA HSP90β for 48 h (J). After adding 50 µM cycloheximide for 1 h, the whole cell extracts were harvested after incubation for indicated periods of time, mSREBPs were detected by immunoblotting with indicated antibodies. The graph depicted the averaged ratio of the autoradiographic signals of flag to actin levels, the dotted lines show the half-life of flag-mSREBP-1/-2. (K) HL-7702 hepatocytes were transfected with indicated siRNA for 48 h. The cells were switched to medium D containing MG-132 for 12 h, the whole cell extracts underwent immunoblotting with indicated antibodies. (L) HL-7702/SRE-Luc cells were transfected with HSP90β siRNAs for 48 h, luciferase activity was then measured. (M) 293T cells were transfected with flag-mSREBP-1 and HA-ubiquitin and with or without siRNA HSP90β and FBW7, ubiquitylated SREBP-1 was detected by immunoblot. (N) HL-7702 hepatocytes were transfected with the indicated siRNA for 48 h, the whole cell extracts underwent immunoblotting with indicated antibodies. Error bars are represented as mean ± SEM. Statistical analyses were done with one-way ANOVA (Dunnett's post test) (A, C, and E). *p < 0.05, **p < 0.01, ***p < 0.001 vs control siRNA or control vector.