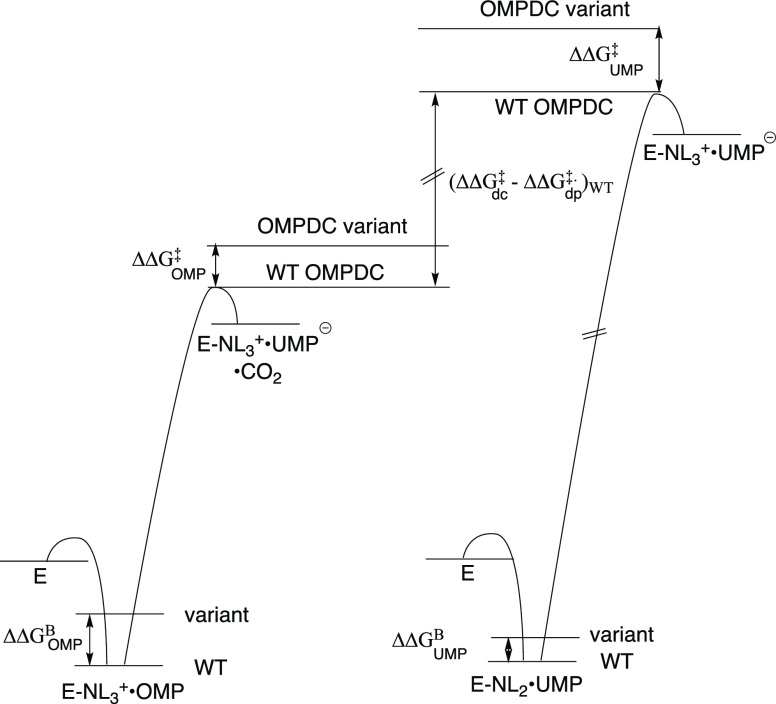
Figure 8.
Hypothetical free-energy profiles for wild type and protein variants of ScOMPDC-catalyzed decarboxylation of OMP and deprotonation of UMP to form UMP vinyl carbanion intermediates UMP-, which are drawn to show the difference in the stabilization of the respective transition states by interactions with the protein catalyst. These profiles were constructed using kinetic data from Table 1, and assuming that the changes in protein structure effect similar changes in the barriers for ScOMPDC-catalyzed deprotonation of FUMP and UMP.6 The diagrams show: (1) The ca. 11 kcal/mol difference in the stabilization of the transition states for wildtype ScOMPDC-catalyzed decarboxylation of OMP (ΔΔG‡)dc and deprotonation of UMP (ΔΔG‡)dp [(ΔΔG‡)dc – (ΔΔG‡)dp = 11 kcal/mol, Figure 7], that we propose is due to stabilization of the former transition state by interactions with the nascent CO2 product. (2) The difference between the effects of Q215A or Y217F substitutions on ΔΔGUMP‡ (relatively large) and ΔΔGOMP [smaller, Table 3]. (3) The larger effect of amino acid substitutions on the stability of the Michaelis complexes to OMP (ΔΔGOMPB) compared with UMP/FUMP (ΔΔGFUMP). The ca 8 kcal/mol barrier to krot, which is required for the deuterium exchange reaction of UMP (kex) but not for decarboxylation of OMP (kdc) or deprotonation of UMP (kdp), is not shown. The barriers to the two enzymatic reactions have not been scaled to show the thermodynamic driving force to decarboxylation of OMP to form UMP, because this driving force is not known.