Abstract
Setting:
The tuberculosis (TB) programmes at the Nonga General Hospital, Rabaul Urban Clinic and Kerevat District Hospital in East New Britain Province, Papua New Guinea.
Background:
In East New Britain, TB care was mainly offered by the General Hospital, resulting in limited community-based care and poor treatment outcomes. In 2016, TB services were decentralised from the provincial to the district level by 1) training health workers, 2) increasing community awareness of TB, and 3) providing a weekly Clinical Outreach (TACO) service.
Objective:
To describe the effect of TACO on the use of TB diagnostic and treatment services from 1 January 2014 to 31 December 2017.
Design:
This was a retrospective study comparing 2014–2015 (pre-TACO) and 2016–2017 (TACO) cohorts.
Results:
There was an increase in pre-TACO to TACO cohorts in screened cases (1581 to 2195), total registered TB cases (678 to 824) and registered cases at decentralised sites (209 to 615). Unfavourable treatment outcomes were common (pre-TACO, 46.0% vs. TACO, 40.1%). In multivariable analysis, treatment at a decentralised Basic Management Unit (aOR 0.55, 95%CI 0.42–0.74) was significantly associated with fewer unfavourable outcomes, but treatment outcomes between the pre-TACO and the TACO group were not significantly different.
Conclusion:
Strengthening decentralisation of TB services at the district level increased TB screening and case registration, with similar treatment outcomes.
Keywords: clinical outreach, district level, community awareness
Abstract
Contexte :
Les programmes de lutte contre la tuberculose (TB) du Nonga General Hospital, de la Rabaul Urban Clinic et du Kerevat District Hospital dans la province d'East New Britain Province, Papouasie Nouvelle Guinée.
Cadre :
En East New Britain, la prise en charge de la TB s'est principalement faite au General Hospital, aboutissant à une prise en charge limitée en communauté et à des résultats médiocres du traitement. A partir de 2016, les services TB ont été décentralisés du niveau provincial au niveau du district grâce à 1) la formation du personnel de santé, 2) la sensibilisation des communautés à la TB, et 3) des stratégies avancées hebdomadaires (TACO).
Objectif :
Décrire l'effet des TACO sur l'utilisation des services de diagnostic et de traitement de TB du 1 janvier 2014 au 31 décembre 2017.
Schéma :
Une étude rétrospective comparant les cohortes 2014–2015 (avant TACO) et 2016–2017 (TACO).
Résultats :
Il y a eu une augmentation des cas dépistés des périodes avant TACO à TACO (1581 à 2195), du total de cas de TB enregistrés (678 à 824) et des cas enregistrés dans des sites décentralisés (209 à 615). Les résultats défavorables du traitement ont été fréquents (46,0% avant TACO contre 40,1% TACO). En analyse multivariable, le traitement dans une unité de base de gestion décentralisée (OR 0,55 ; IC95% 0,42–0,74) a été significativement associé à moins de résultats défavorables, mais les résultats du traitement dans les périodes avant TACO et TACO n'ont pas été significativement différentes.
Conclusion :
Le renforcement de la décentralisation des services TB au niveau du district a augmenté le dépistage de la TB et l'enregistrement des cas avec des résultats de traitement similaires.
Abstract
Marco de Referencia:
Los programas contra la tuberculosis (TB) en el Hospital General de Nonga, el consultorio urbano de Rabaul y el Hospital Distrital de Kerevat de la Provincia de Nueva Bretaña Oriental en Papúa Nueva Guinea.
Marco de Referencia:
En la Provincia de Nueva Bretaña Oriental la atención de la TB se presta en su mayor parte en el Hospital General, con lo cual la atención a nivel de la comunidad es deficiente y los desenlaces clínicos son desfavorables. A partir del 2016 se descentralizaron los servicios de TB del nivel provincial hacia el nivel distrital con intervenciones de 1) capacitación de los profesionales de salud; 2) sensibilización comunitaria a la TB; y 3) actividades médicas extrainstitucionales semanales (TACO).
Objetivo:
Describir el efecto de la iniciativa TACO sobre la utilización de los servicios de diagnóstico y tratamiento de la tuberculosis del 1 de enero del 2014 al 31 de diciembre del 2017.
Método:
Fue este un estudio comparativo de cohortes retrospectivo de 2014–2015 (pre-TACO) y 2016–2017 (TACO).
Resultados:
Se observó un aumento de los casos investigados por TB del período pre-TACO al período de la intervención (de 1581 a 2195), un mayor número de casos de TB registrados (de 678 a 824) y de casos registrados en los centros descentralizados (de 209 a 615). Los desenlaces desfavorables fueron frecuentes (46,0% en período pre-TACO contra 40,1% en el TACO). En el análisis multivariante, el tratamiento en las unidades básicas de gestión descentralizadas se asoció de manera significativa con menos desenlaces desfavorables (OR 0,55; IC95% 0,42–0,74), pero no se observó ninguna diferencia entre los períodos de intervención.
Conclusión:
El fortalecimiento de la descentralización de los servicios de atención de la TB hacia el nivel distrital aumentó la práctica de la detección de la TB y el registro de casos, pero no modificó de manera importante los desenlaces clínicos.
Tuberculosis (TB) is a leading cause of hospital admissions and death in Papua New Guinea (PNG), with an estimated incidence of 417 per 100 000 population.1 The current National Strategic Plan for TB in PNG is implemented through 275 Basic Management Units (BMUs) in 89 districts.2 BMUs are health clinics that ensure TB cases are diagnosed, registered and treated. However, less than half of the BMUs have TB diagnostic testing capacity.2 National TB treatment outcomes continue to remain below target at 64% success rate, with 19% loss to follow-up (LTFU) reported in 2016.3 Health management deficiencies have prevented effective implementation of TB control activities even where resources have been sufficient.4 Within provinces, many patients are bypassing local facilities in favour of provincial hospitals.4 Facility-based care, with little attention to patient adherence support close to where patients live, likely contributes to the high LTFU reported in PNG.4
In East New Britain Province, most TB care is offered at the provincial hospital, Nonga General Hospital (NGH), Rabaul.5 In 2016, infrastructural issues at NGH resulted in the redirection of people seeking TB services back to their local district facilities. To promote greater use and quality of those district facilities, a service delivery package comprising integrated specialist medical and TB clinical support was rolled out at two BMUs at the district level to complement planned TB training among primary health care workers. Public awareness campaigns about early symptoms of TB and the availability of specialist consultation at the two district facilities were also conducted throughout the year.
The present operational research study examined the combined effect of ‘Training, Awareness and Clinical Outreach’ (TACO) on the use of TB diagnostic and treatment services and evaluated factors associated with unfavourable treatment outcomes in three BMUs in East New Britain, PNG.
METHODS
Study design and participants
This was a retrospective cohort study of presumptive TB cases and registered TB cases at three facilities in East New Britain. A comparison was performed between two periods: 1 January 2014–31 December 2015 (pre-TACO) and 1 January 2016–31 December 2017 (TACO). All patients recorded in the TB laboratory registers as evaluated for diagnosis (presumptive TB cases) and all patients registered in the TB treatment registers during the two time periods were eligible for inclusion. In comparing treatment outcomes, those cases registered after 30 June 2017 were excluded from the analysis.
Study setting
East New Britain Province is an island province in PNG with a population of over 270 000.6 It has 14 TB BMUs, but half of all TB notifications come from three BMUs: the centralised NGH BMU, and two decentralised BMUs (Kerevat Rural Hospital, Kerevat, and Rabaul Urban Clinic, Rabaul).5 In 2015, 81% of patients registered at the NGH TB clinic came from outside of its catchment area, with 54% living in the catchments of the two decentralised BMUs.5
NGH is a provincial hospital and offers microscopy, Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) and chest radiography, and provides treatment for drug-susceptible and drug-resistant TB with care from a combined team of physicians, health extension officers, nursing officers and community health workers. The two decentralised BMUs are located approximately 5.4 km and 24 km from NGH. TB services in the decentralised BMUs include sputum smear microscopy and drug-susceptible TB treatment; however, cases requiring X-ray and sputum samples for Xpert are referred to NGH. In accordance with the national TB treatment protocol, TB diagnosis and treatment are provided free of cost.7 All three BMUs offer on-site counselling, testing and treatment for human immunodeficiency virus (HIV) infection. The TB case finding model was passive and facility-based, with self-administered treatment.
All facilities maintained laboratory registers on TB diagnostic activity and TB registers to record treatment indicators in those registered at the facility. With dedicated disease control transportation and the support of the Internal Medicine Unit, the NGH was able to implement a home-based model for bacteriologically confirmed pulmonary TB cases, where clinical review, sputum collection and resupply of medicines were conducted during home visits from February 2015 to March 2016. Child TB services remain underdeveloped, and only after the 2016 training was there an emphasis on facility-based screening of child contacts for the purpose of isoniazid preventive therapy (IPT) at the BMU level. There were several changes in PNG TB management protocols during the study period, including the use of Xpert for screening all presumptive TB cases (this was routinely performed at the NGH only), introduction of out-patient registers for presumptive TB, discontinuation of streptomycin use, reclassification of treatment categories and introduction of new reporting formats.
Training, awareness and clinical outreach
From April to November 2016, the TB Clinic at NGH underwent emergency renovation and patients were directed to strengthen the decentralised BMUs. The NGH Internal Medicine Unit initiated weekly integrated clinical TB and specialist medical consultation outreach services to district facilities in 2016–2017. Weekly outreach involved patient review, treatment card review and visiting at home sputum smear-positive cases who missed an appointment. Health education would be routinely provided to clients by the nurse while waiting for the physician to arrive. The unit concurrently conducted a public awareness campaign comprising health messages to raise awareness of early symptoms suggestive of TB and encourage attendance at the BMUs. This included sessions with schools, churches and community groups, and a weekly (provincial) radio programme about TB and where to access TB services. Structured training courses on TB management (March 2016), HIV provider-initiated testing and counselling (July 2016) and integrated management of adult illness (September 2016) were conducted for health workers in district facilities, including the two decentralised BMUs.8,9
Data collection
Demographic and clinical data were collected from the TB laboratory registers and TB treatment registers at all three facilities and entered into two separate databases using EpiData v 3.1 (EpiData Association, Odense, Denmark). Entries in the laboratory register marked as ‘New’ and ‘Diagnostic’ were considered to be ‘presumptive TB cases’. All data were double-entered and validated. The travel time to the BMUs was estimated by a transport official and defined as the estimated time taken for a round trip from the ward to the BMU. Each address was assigned to one of three categories of travel time to BMU: <3 hours, ⩾3 hours and unknown.
We used the standard definitions of treatment outcomes of cured, completed treatment, LTFU, died, treatment failure and not evaluated as defined in the PNG TB guidelines.7 Unfavourable outcomes included LTFU, treatment failure, not evaluated and died. Favourable outcomes included cured and treatment completed. Paediatric cases were defined as any patients aged ⩽14 years.
Data analysis and statistics
Data were analysed using Stata v 15 (StataCorp, College Station, TX, USA). Categorical variables were reported as numbers and proportions. Age was reported as median with interquartile ranges [IQR]. Categorical variables were analysed using the χ2 test. To assess whether TACO improved treatment outcomes, risk factors for unfavourable outcomes were identified using univariable and multivariable logistic regression and expressed as odds ratio (ORs) and 95% confidence interval (CIs). Levels of significance were set at 5%.
Ethics approval
Ethics approval was provided by the PNG Medical Research Advisory Council (MRAC), Port Moresby, PNG and the Alfred Hospital Ethics Committee, Melbourne, VIC, Australia.
RESULTS
A total of 3776 patients with presumptive TB were screened at the health facilities between 1 January 2014 and 31 December 2017: 1581 were screened pre-TACO and 2195 screened during TACO (Table 1). The number of children screened increased from the pre-TACO to the TACO period (120/1581, 7.6% vs. 299/2195, 13.6%; P < 0.001).
TABLE 1.
Patients with presumptive TB investigated during the pre-TACO and TACO period in East New Britain Province, Papua New Guinea, 2014–2017
| Pre-TACO January 2014–December 2015 (n = 1581) n (%) | TACO January 2016–December 2017 (n = 2195) n (%) | Total (n = 3776) n (%) | P value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years, median [IQR]* | 35 [24–50] | 34 [21–50] | 34 [22–50] | 0.009 |
| Age group, years | ||||
| <15 | 120 (7.6) | 299 (13.6) | 419 (11.1) | <0.001 |
| ⩾15 | 1356 (85.8) | 1795 (81.8) | 3151 (83.4) | |
| Unknown† | 105 (6.6) | 101 (4.6) | 206 (5.5) | |
| Sex | ||||
| Female | 814 (51.5) | 1095 (49.9) | 1909 (50.6) | 0.395 |
| Male | 756 (47.8) | 1076 (49.0) | 1832 (48.5) | |
| Unknown† | 11 (0.7) | 24 (1.1) | 35 (0.9) | |
| BMU location | ||||
| Centralised | 1275 (80.6) | 1697 (77.3) | 2972 (78.7) | 0.014 |
| Decentralised | 306 (19.4) | 498 (22.7) | 804 (21.3) | |
| Distance to BMU | ||||
| <3 h | 924 (58.4) | 1219 (55.5) | 2143 (56.8) | 0.007 |
| >3 h | 539 (34.1) | 859 (39.1) | 1398 (37.0) | |
| Unknown† | 118 (7.5) | 117 (5.3) | 235 (6.2) | |
| Diagnostic investigations | ||||
| Smear microscopy | ||||
| Positive | 263 (16.6) | 325 (14.8) | 588 (15.6) | 0.336 |
| Negative | 1292 (81.7) | 1742 (79.4) | 3034 (80.3) | |
| Missing† | 26 (1.6) | 128 (5.8) | 154 (4.1) | |
| Xpert status | ||||
| M. tuberculosis not detected | 32 (2.0) | 564 (25.7) | 596 (15.8) | ‡ |
| M. tuberculosis, no RMP resistance | 15 (0.9) | 141 (6.4) | 156 (4.1) | |
| M. tuberculosis, RMP resistance detected | 8 (0.5) | 8 (0.4) | 16 (0.4) | |
| Missing | 1526 (96.5) | 1482 (67.5) | 3008 (79.7) | |
* Missing data: pre-TACO, n = 1476; TACO, n = 2094.
† Variables with an unknown/missing category <10% of the total sample were excluded from the χ2 tests.
‡ Not calculated for this variable due to the large proportion of missing Xpert results.
TB = tuberculosis; TACO = Training, Community Awareness and Clinical Outreach; IQR = interquartile range; BMU = Basic Management Unit; RMP = rifampicin.
A total of 1502 TB patients were registered for anti-tuberculosis treatment, 678 in the pre-TACO period and 824 during TACO (Table 2). In addition, there were 21 patients with reported rifampicin-resistant TB (8 pre-TACO and 13 TACO), who were excluded from the analysis. The pre-TACO cohort had a higher median age than the TACO cohort (28 [IQR 17–41] vs. 22 years [IQR 7–36]; P < 0.001) and a lower proportion of registered patients aged <15 years (140/678, 20.6% vs. 295/824, 35.8%; P < 0.001). Compared to the pre-TACO cohort, the TACO cohort had a higher proportion of 1) patients registered in the decentralised BMUs (209/678, 30.8% vs. 615/824, 74.6%; P < 0.001); 2) extra-pulmonary TB (EPTB) patients (183/678, 27.0% vs. 315/824, 38.2%; P < 0.001); and 3) known HIV status (81/678, 11.9% vs. 180/824, 21.8%; P < 0001).
TABLE 2.
Demographic and clinical characteristics of registered TB cases during the pre-TACO and TACO period in East New Britain Province, Papua New Guinea, 2014–2017
| Pre-TACO January 2014–December 2015 (n = 678) n (%) | TACO January 2016–December 2017 (n = 824) n (%) | Total (n = 1502) n (%) | P value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years, median [IQR]* | 28 [17–41] | 22 [7–36] | 25 [10–39] | <0.001 |
| Age group, years | ||||
| <15 | 140 (20.6) | 295 (35.8) | 435 (29) | <0.001 |
| ⩾15 | 530 (78.2) | 523 (63.5) | 1035 (70.1) | |
| Unknown† | 8 (1.2) | 6 (0.7) | 14 (0.9) | |
| Sex | ||||
| Female | 346 (51.0) | 396 (48.1) | 742 (49.4) | 0.24 |
| Male | 331 (48.8) | 428 (51.9) | 759 (50.5) | |
| Unknown† | 1 (0.1) | 0 | 1 (0.1) | |
| BMU location | ||||
| Centralised | 469 (69.2) | 209 (25.4) | 678 (45.1) | <0.001 |
| Decentralised | 209 (30.8) | 615 (74.6) | 824 (54.9) | |
| Distance to BMU | ||||
| <3 h | 532 (78.5) | 624 (75.7) | 1156 (77.0) | 0.22 |
| >3 h | 143 (21.1) | 195 (23.7) | 338 (22.5) | |
| Unknown† | 3 (0.4) | 5 (0.6) | 8 (0.5) | |
| Clinical characteristics | ||||
| Site of TB | ||||
| Pulmonary TB | 485 (71.5) | 501 (60.8) | 986 (65.6) | <0.001 |
| Extra-pulmonary TB | 183 (27.0) | 315 (38.2) | 498 (33.2) | |
| Missing† | 10 (1.5) | 8 (1.0) | 18 (1.2) | |
| Type of TB | ||||
| New | 544 (80.2) | 702 (85.2) | 1246 (83.0) | <0.001 |
| Previously Treated | 122 (18.0) | 92 (11.2) | 214 (14.2) | |
| Missing† | 12 (1.8) | 30 (3.6) | 42 (2.8) | |
| HIV status | ||||
| Negative | 53 (7.8) | 141 (17.1) | 194 (12.9) | <0.001 |
| Positive | 28 (4.1) | 39 (4.7) | 67 (4.5) | |
| Missing | 597 (88.1) | 644 (78.2) | 1241 (82.6) | |
| Smear status | ||||
| Negative | 157 (23.2) | 131 (15.9) | 288 (19.2) | <0.001 |
| Positive | 191 (28.2) | 192 (23.3) | 383 (25.5) | |
| Missing | 330 (48.7) | 501 (60.8) | 831 (55.3) | |
| Xpert status | ||||
| M. tuberculosis not detected | 9 (1.3) | 10 (1.2) | 19 (1.3) | ‡ |
| M. tuberculosis, no RMP resistance | 11 (1.6) | 33 (4.0) | 44 (2.9) | |
| M. tuberculosis, RMP resistance detected | 5 (0.7) | 1 (0.1) | 6 (0.4) | |
| Missing | 653 (96.3) | 780 (94.7) | 1433 (95.4) | |
* Missing data: pre-TACO, n = 670; TACO, n = 818.
† Variables with an unknown/missing category <10% of the total sample were excluded from the χ2 tests.
‡ Not calculated for this variable due to the large proportion of missing Xpert results.
TB = tuberculosis; TACO = Training, Community Awareness and Clinical Outreach; IQR = interquartile range; BMU = Basic Management Unit; HIV = human immunodeficiency virus; RMP = rifampicin.
Patients registered after 30 June 2017 (n = 205) were excluded from outcome analysis, as most were still on treatment at the time of the study. Overall treatment outcomes were similar between the two cohorts (Figure 1). There was an increase in the treatment success rate (366/678, 54.0% vs. 371/619, 60%; P = 0.031) and lower LTFU rate (236/678, 34.8% vs. 179/619, 28.9%; P = 0.019) from the pre-TACO to the TACO period.
FIGURE.
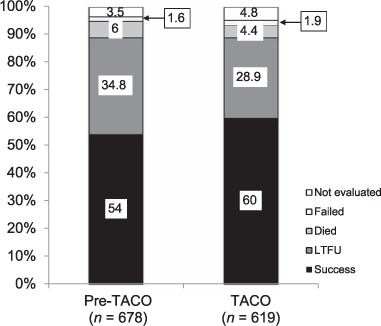
TB treatment outcomes during the pre-TACO and TACO periods in East New Britain Province, Papua New Guinea, 2014–2017. TACO = Training, Community Awareness and Clinical Outreach; TB = tuberculosis; LTFU = lost to follow-up.
The proportion of unfavourable outcomes was 46% (312/678) in the pre-TACO period, and 40.1% (248/619) in the TACO period. Univariable and multivariable associations with unfavourable outcomes are presented in Table 3. On univariable analysis, registration in the TACO period (OR 0.78, 95%CI 0.63–0.98) and treatment at a decentralised BMU (OR 0.64, 95%CI 0.52–0.80) were significantly associated with fewer unfavourable outcomes. In multivariable analysis, treatment at a decentralised BMU (aOR 0.55, 95%CI 0.42–0.74) was significantly associated with fewer unfavourable outcomes, but treatment outcomes in the pre-TACO and TACO periods were not significantly different (aOR 1.14, 95%CI 0.86–1.51).
TABLE 3.
Risk factors for unfavourable outcomes among TB cases registered in East New Britain Province, Papua New Guinea, 1 January 2014–1 July 2017 *
| Favourable outcome (n = 737) n (%) | Unfavourable outcome (n = 560) n (%) | Total (n = 1297) n | OR (95%CI) | aOR (95%CI) | |
|---|---|---|---|---|---|
| Programmatic characteristics | |||||
| TACO | |||||
| Pre-TACO | 366 (54.0) | 312 (46.0) | 678 | Reference | Reference |
| TACO | 371 (59.9) | 248 (40.1) | 619 | 0.78† (0.63–0.98) | 1.14 (0.86–1.51) |
| BMU | |||||
| Centralised | 305 (51.0) | 293 (49.0) | 598 | Reference | Reference |
| Decentralised | 432 (61.8) | 267 (38.2) | 699 | 0.64† (0.52–0.80) | 0.55† (0.42–0.74) |
| Demographic characteristics | |||||
| Age, years | |||||
| <15 | 192 (56.5) | 148 (43.5) | 340 | Reference | Reference |
| ⩾15 | 537 (56.9) | 406 (43.1) | 943 | 0.98 (0.76–1.26) | 1.22 (0.91–1.65) |
| Unknown | 8 (57.1) | 6 (42.9) | 14 | — | — |
| Sex | |||||
| Female | 385 (59.8) | 259 (40.2) | 644 | Reference | Reference |
| Male | 351 (53.8) | 301 (46.2) | 652 | 1.27† (1.02–1.59) | 1.21 (0.96–1.52) |
| Unknown | 1 (100) | 0 | 1 | — | — |
| Distance to BMU | |||||
| <3 h | 591 (57.9) | 429 (42.1) | 1,020 | Reference | Reference |
| >3 h | 139 (51.5) | 131 (48.5) | 270 | 1.30 (1.0–1.70) | 1.35† (1.02–1.79) |
| Unknown | 7 (100) | 0 | 7 | — | — |
| Bacteriological characteristics | |||||
| Type of TB | |||||
| Confirmed PTB | 226 (65.3) | 120 (34.7) | 346 | Reference | Reference |
| Probable PTB‡ | 301 (57.0) | 227 (43.0) | 528 | 1.42† (1.07–1.88) | 1.51† (1.22–2.04) |
| Extra-pulmonary TB | 206 (50.6) | 201 (49.4) | 407 | 1.84† (1.37–2.47) | 1.95† (1.39–2.74) |
| Missing | 4 (25.0) | 12 (75.0) | 16 | — | — |
| Registration category | |||||
| New | 620 (57.7) | 455 (42.3) | 1,075 | Reference | Reference |
| Retreatment | 103 (51.8) | 96 (48.2) | 199 | 1.27 (0.94–1.72) | 1.47† (1.07 – 2.03) |
| Missing | 14 (60.9) | 19 (39.1) | 23 | — | — |
| HIV status | |||||
| Negative | 115 (68.5) | 53 (31.5) | 168 | Reference | Reference |
| Positive | 35 (54.7) | 29 (45.3) | 64 | 1.80 (1.00–3.24) | 1.56 (0.84–2.90) |
| Missing | 585 (55.0) | 480 (45.0) | 1065 | 1.77† (1.25–2.51) | 1.87† (1.27–2.76) |
*Categories with <10% of total sample of missing data were left out of the univariable and multivariable analyses.
† P < 0.05.
‡ Clinically diagnosed PTB.
TB = tuberculosis; OR = odds ratio; CI = confidence interval; aOR = adjusted odds ratio; TACO = Training, Community Awareness and Clinical Outreach; BMU = Basic Management Unit; PTB = pulmonary TB; HIV = human immunodeficiency virus.
DISCUSSION
Integrated clinical support to decentralised BMUs led to increased numbers of patients with presumptive TB being screened and TB cases being registered; however, this did not significantly improve treatment outcomes. Decentralising TB services has been reported to result in increased case notification rates in other settings and is cost-effective.10–14 Having strong centralised programmes at provincial hospitals, as is the case in East New Britain, may encourage people to bypass more accessible district facilities.15,16 The distance and cost of travel to health services are important determinants of health-seeking behaviour and may lead to delays.17–20 Further decentralisation of TB services to the community (ward) level could increase access to services and case finding, and improve treatment outcomes.
Despite improvement, treatment outcomes in the TACO group remained well short of national targets. LTFU remained high despite the use of TACO, which suggests that proximity of services alone is not enough to improve outcomes. Decentralised treatment is associated with higher treatment success and lower LTFU.13,21 Programme factors associated with improved treatment outcomes include patient and health care provider education, inclusion of incentives and enablers, psychological counselling and support, patient tracing for missed appointments, and reminders, including the use of mobile digital technologies.21 In PNG, reported barriers to TB treatment adherence and completion include distance to treatment sites and the need to maintain subsistence food production, while a high confidence in the anti-tuberculosis treatment can be a positive influence.15 In our study, the proportion of patients residing at a distance of >3 h from the BMU remained similar in the two time periods, suggesting that the decentralisation of services may be allowing people from even more remote areas to access services. Distance to BMU was significantly associated with unfavourable outcomes, suggesting further decentralisation of TACO to the community level (ward or village), and the provision of patient tracing and enablers, in particular, may help improve treatment outcomes.
The increased number of paediatric cases in TACO was probably due to the increased focus on screening child contacts and the expansion of IPT. This increase may account for the increased proportions of new and EPTB cases, and suggests that easier access to TB services made families more likely to bring their children to the health facility. The overall proportion of paediatric TB among registered cases was high, at 29%. While this could represent overdiagnosis, paediatric TB is reported to comprise 20–30% of cases in all provinces in PNG, and accounts for a high proportion of paediatric admissions and mortality.3,22 Although often specialised, paediatric TB services can be effectively implemented at a decentralised level.23
Community-based, patient-centred services are recommended for TB management.24 In the BMUs studied, the model of care, TACO, was self-administered treatment complemented with patient education and home visits. There were some programmatic constraints that disrupted home visits, resulting in this particular component not being uniformly implemented over the study period between the three facilities, which may have impacted on LTFU rates. These constraints included non-availability of transport, multitasking at the district-level facilities, gaps in human resources, and the occasional (although infrequent) drug stock-out. In addition, bacteriological confirmation remained low even after the introduction of Xpert. There is a need for improved understanding of appropriate resource allocation, training in sputum transport logistics and supervision for the successful implementation of the community-based treatment model for TB in rural PNG.
All confirmed HIV-positive patients were enrolled on antiretroviral treatment at their local BMU. HIV testing improved in the TACO period, but remained low. This is a major concern throughout PNG, where only one third of registered TB patients in 2016 had a known status.1 Unknown HIV status was also associated with unfavourable outcomes, and may be a reflection of undiagnosed and untreated HIV infection in persons with TB. There were constant shortages of HIV rapid testing kits throughout the entire period of the study. Attention to training, supervision of stock management and the introduction of stock reporting systems using simple SMS has resulted in reductions of stock-outs in other contexts and should be reviewed in PNG.25,26
This was a retrospective cohort study with limitations due to the routinely collected programme data. The first 6 months of the TACO period saw the introduction over time of the whole TACO package, and inclusion of data from this period may have diluted the measured impact of TACO. Other factors likely influenced TB service utilisation and outcomes, including the temporary closure of provincial hospital TB clinics, stock-outs of HIV testing kits and the introduction of Xpert in late 2014. Presumptive cases did not include all patients investigated for possible TB without productive cough, or those with EPTB and cannot be used to describe any changes in the presentation of children for TB investigation. Despite these limitations, the use of operational research to examine changes during the study period provide useful and generalisable (as the study setting is similar to many parts of the country) information on the effects of decentralisation of TB services in PNG.
CONCLUSION
The TACO model of decentralising TB services from provincial hospitals to district facilities increased clinical attendance at TB diagnostic and treatment services in PNG. More locally designed interventions need to be developed to further promote community-based, patient-centred care in the face of ongoing resource and health system constraints in order to improve TB treatment outcomes and promote equitable access to health services.
Acknowledgments
This research was conducted as part of the first Operational Research Course for Tuberculosis in Papua New Guinea (PNG). The specific training programme that resulted in this publication was developed and implemented by the Burnet Institute (Melbourne, VIC, Australia) in collaboration with the PNG Institute of Medical Research (Goroka) and University of PNG (Port Moresby), and supported by the PNG National Department of Health Emergency Response Taskforce for MDR and XDR-TB, the National TB Programme and Western Provincial Health Office, Daru, PNG. The model is based on the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR).
The authors thank the following people and institutions that contributed to the programme under which this operational research was conducted: K Witari, J Schulz, H Benson, M Lolo (East New Britain Provincial Administration, Nonga General Hospital, Rabaul); M Kanini (Rabaul Urban Clinic, Rabaul); R Ruga (Kerevat Rural Hospital, Kerevat, PNG); and health workers and pathology staff in all three facilities for their support.
The training programme was delivered as part of the Tropical Disease Research Regional Collaboration Initiative, which is supported by the Australian Government and implemented by Menzies School of Health Research (Darwin, NT, Australia) and the Burnet Institute.
The views expressed in this publication are the authors' alone and are not necessarily the views of the Australian or PNG Governments. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
References
- 1.Frieden T R, Brudney K F, Harries A. Global tuberculosis: perspectives, prospects, and priorities. JAMA. 2014;312:1393–1394. doi: 10.1001/jama.2014.11450. [DOI] [PubMed] [Google Scholar]
- 2.Papua New Guinea Government National Tuberculosis Strategic Plan (NSP) for Papua New Guinea. Port Moresby, PNG: PNG Government; 2015. [Google Scholar]
- 3.Aia P, Wangchuk L, Morishita F et al. Epidemiology of tuberculosis in Papua New Guinea : analysis of case notification and treatment outcome data, 2008–2016. Western Pac Surveill Response J. 2018;9:9–19. doi: 10.5365/wpsar.2018.9.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmichael H, Mola G, Amos L, Wemin J, Majumdar P, Matheson D. The mid-term review and joint assessment of the Papua New Guinea National Health Plan 2011–2020. Port Moresby, Papua New Guinea: PNG Government; 2015. [Google Scholar]
- 5.East New Britain Provincial Administration East New Britain provincial tuberculosis patient database. Kokopo, PNG: East New Britain Provincial Administration; 2015. [Google Scholar]
- 6.National Statistical Office Papua New Guinea 2011 national population & housing census ward population profile – islands region. Port Moresby, Papua New Guinea: NSO; 2014. [Google Scholar]
- 7.National Department of Health Papua New Guinea Papua New Guinea national tuberculosis management protocol. Port Moresby, Papua New Guinea: NDH; 2016. [Google Scholar]
- 8.World Health Organization Guidance on provider-initiated HIV testing and counselling in health facilities. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 9.World Health Organization Acute care: integrated management of adolescent and adult illness (IMAAI) Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 10.Sinanovic E, Floyd K, Dudley L, Azevedo V, Grant R, Maher D. Cost and cost-effectiveness of community-based care for tuberculosis in Cape Town, South Africa. Int J Tuberc Lung Dis. 2003;7(Suppl 1):S56–S62. [PubMed] [Google Scholar]
- 11.Floyd K, Skeva J, Nyirenda T, Gausi F, Salaniponi F. Cost and cost-effectiveness of increased community and primary care facility involvement in tuberculosis care in Lilongwe District, Malawi. Int J Tuberc Lung Dis. 2003;7(Suppl 1):S29–S37. [PubMed] [Google Scholar]
- 12.Kangangi J K, Kibuga D, Muli J et al. Decentralisation of tuberculosis treatment from the main hospitals to the peripheral health units and in the community within Machakos district, Kenya. Int J Tuberc Lung Dis. 2003;7(Suppl 1):S5–S13. [PubMed] [Google Scholar]
- 13.Wei X, Liang X, Liu F, Walley J D, Dong B. Decentralising tuberculosis services from county tuberculosis dispensaries to township hospitals in China: an intervention study. Int J Tuberc Lung Dis. 2008;12:538–547. [PubMed] [Google Scholar]
- 14.Méda Z C, Huang C C, Sombié I et al. Tuberculosis in developing countries: conditions for successful use of a decentralized approach in a rural health district. Pan Afr Med J. 2014;17:198. doi: 10.11604/pamj.2014.17.198.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diefenbach-Elstob T, Plummer D, Dowi R et al. The social determinants of tuberculosis treatment adherence in a remote region of Papua New Guinea. BMC Public Health. 2017;17:1–12. doi: 10.1186/s12889-016-3935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgins S, Pullum T, Dougherty L. Understanding where parents take their sick children and why it matters: a multicountry analysis. Glob Heal Sci Pract. 2013;1:328–356. doi: 10.9745/GHSP-D-13-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feikin D R, Nguyen L M, Adazu K et al. The impact of distance of residence from a peripheral health facility on pediatric health utilisation in rural western Kenya. Trop Med Int Health. 2009;14:54–61. doi: 10.1111/j.1365-3156.2008.02193.x. [DOI] [PubMed] [Google Scholar]
- 18.Bertakis K D, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24:229–239. doi: 10.3122/jabfm.2011.03.100170. [DOI] [PubMed] [Google Scholar]
- 19.Anselmi L, Lagarde M, Hanson K. Health service availability and health seeking behaviour in resource poor settings: evidence from Mozambique. Health Econ Rev. 2015;5:62. doi: 10.1186/s13561-015-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller I, Smith T, Mellor S, Rare L, Genton B. The effect of distance from home on attendance at a small rural health centre in Papua New Guinea. Int J Epidemiol. 27:878–884. doi: 10.1093/ije/27.5.878. [DOI] [PubMed] [Google Scholar]
- 21.Alipanah N, Jarlsberg L, Miller C et al. Adherence interventions and outcomes of tuberculosis treatment : a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002595. e1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watch V, Aipit J, Kote-yarong T et al. The burden of presumed tuberculosis in hospitalized children in a resource-limited setting in Papua New Guinea: a prospective observational study. Int Health. 2017;9:374–378. doi: 10.1093/inthealth/ihx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zawedde-Muyanja S, Nakanwagi A, Dongo J P et al. Decentralisation of child tuberculosis services increases case finding and uptake of preventive therapy in Uganda. Int J Tuberc Lung Dis. 2018;22:1314–1321. doi: 10.5588/ijtld.18.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization Implementing the End TB Strategy: the essentials. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 25.Githinji S, Kigen S, Memusi D et al. Reducing stock-outs of life saving malaria commodities using mobile phone text-messaging: SMS for life study in Kenya. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054066. e54066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treatment Action Campaign, Médecins Sans Frontières, Rural Health Advocacy Project, Rural Doctors Association of South Africa, Section 27, Southern African HIV Clinicians Society Stock outs in South Africa: Second Annual Report. Johannesburg, South Africa: TAC; 2015. [Google Scholar]


