Figure 8.
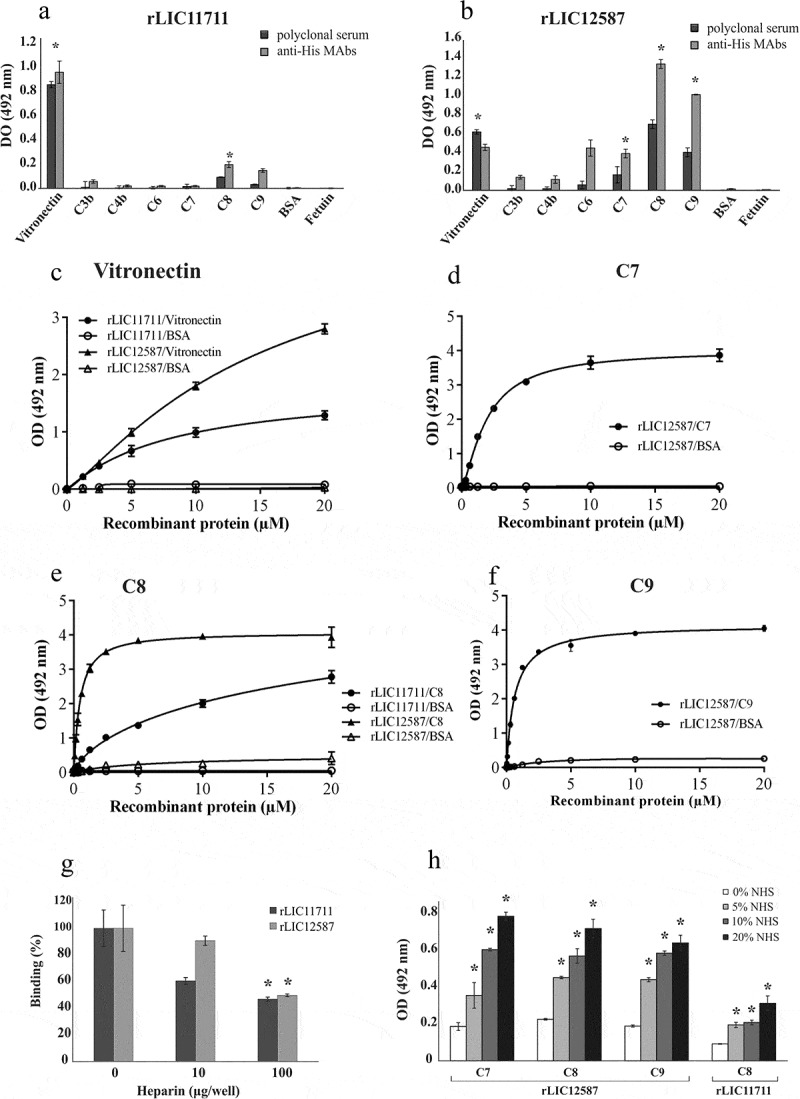
Binding of recombinant proteins with human complement system. The distinct components and the negative control proteins BSA and fetuin were adsorbed into the wells and incubated with 1 μg/well of (a) rLIC11711 or (b) rLIC12587. For binding detection, the anti-His MAbs or antiserum against recombinant protein plus HRP-conjugated anti-mouse were used. Representative results refer to one independent experiment out of two and bars represent mean ± SD of an experimental triplicate. For statistical analysis, the binding of recombinant proteins was compared to their binding to fetuin and BSA by Student’s t-test. For each statistically significant binding, a dose-response assay was performed with (c) vitronectin, (d) C7, (e) C8 and (f) C9. Components and negative control BSA were immobilized and increasing concentrations of recombinant protein were added to wells. Bound proteins were detected with anti-His mAbs. Each point of the curve represents a triplicate and the value was expressed as the mean ± SD. (g) The vitronectin was immobilized and incubated with the recombinant protein plus increasing amounts of heparin; binding was detected incubating anti-His mAbs. For statistical analysis, binding of recombinant proteins to vitronectin was compared to treatment without heparin by Student’s t-test. (h) Capture of complement components from NHS by recombinant proteins. Recombinant proteins were immobilized and incubated with increasing concentrations of NHS. The interaction with complement components was assessed by the addition of specific antibody against each component plus HRP-conjugated secondary antibody. Absorbance represents the mean ± SD of a triplicate. For statistical analysis, acquisition of complement by recombinant proteins was compared to treatment without NHS by Student’s t-test (*p < 0.05).
