Abstract
Phosphate homeostasis is critical for many cellular processes and is tightly regulated. The sodium-dependent phosphate cotransporter, NaPi2a, is the major regulator of urinary phosphate reabsorption in the renal proximal tubule. Its activity is dependent upon its brush border localization that is regulated by fibroblast growth factor 23 (FGF23) and PTH. High levels of FGF23, as are seen in the Hyp mouse model of human X-linked hypophosphatemia, lead to renal phosphate wasting. Long-term treatment of Hyp mice with 1,25-dihydroxyvitamin D (1,25D) or 1,25D analogues has been shown to improve renal phosphate wasting in the setting of increased FGF23 mRNA expression. Studies were undertaken to define the cellular and molecular basis for this apparent FGF23 resistance. 1,25D increased FGF23 protein levels in the cortical bone and circulation of Hyp mice but did not impair FGF23 cleavage. 1,25D attenuated urinary phosphate wasting as early as one hour postadministration, without suppressing FGF23 receptor/coreceptor expression. Although 1,25D treatment induced expression of early growth response 1, an early FGF23 responsive gene required for its phosphaturic effects, it paradoxically enhanced renal phosphate reabsorption and NaPi2a protein expression in renal brush border membranes (BBMs) within one hour. The Na-H+ exchange regulatory factor 1 (NHERF1) is a scaffolding protein thought to anchor NaPi2a to the BBM. Although 1,25D did not alter NHERF1 protein levels acutely, it enhanced NHERF1-NaPi2a interactions in Hyp mice. 1,25D also prevented the decrease in NHERF1/NaPi2a interactions in PTH-treated wild-type mice. Thus, these investigations identify a novel role for 1,25D in the hormonal regulation of renal phosphate handling.
X-linked hypophosphatemia (XLH) is the most prevalent human inherited phosphate wasting disorder. In XLH and its murine homologue, the Hyp mouse, inactivating mutations of the phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) lead to high fibroblast growth factor 23 (FGF23) levels (1). FGF23 lowers serum phosphate levels by downregulating the renal sodium-dependent phosphate cotransporter 2a (NaPi2a). FGF23 also impairs 1,25-dihydroxyvitamin D (1,25D) production by decreasing CYP27B1, the vitamin D 1-α hydroxylase, in renal proximal tubular cells (PTCs) (2, 3). The XLH phenotype includes rickets in growing children/animals and osteomalacia. Although PHEX is expressed in mature osteoblasts, osteocytes, and odontoblasts, the exact physiological function of PHEX and how mutations in this gene result in enhanced FGF23 synthesis are still unknown (4). Studies using osteocalcin-creatinine–mediated PHEX deletion demonstrate that impairing PHEX function in osteoblasts/osteocytes is sufficient to recapitulate the biochemical and skeletal phenotype of XLH (5).
An FGF receptor-αklotho complex mediates the renal effects of FGF23. Studies in mouse models with FGF receptor deletions demonstrate that FGFR1 is the principal mediator of FGF23-induced phosphaturia with FGFR3 and 4 having minor roles (6, 7). FGF23 acutely induces phosphorylation of ERK1/2 in normal mice and upregulates early growth response 1 (EGR1) mRNA expression as early as one hour (8, 9). Studies in EGR1 knockout mice confirm the role of this protein in FGF23-mediated inhibition of renal phosphate transport (9).
Renal brush border sodium-dependent phosphate cotransporter (NaPi2a) expression requires interactions of its C-terminal region with the scaffolding protein Na/H exchange regulatory factor 1 (NHERF1), which in turn interacts with cytoskeletal proteins (10). The importance of NHERF1 in phosphate homeostasis is supported by studies in NHERF1 knockout mice (11) and by data demonstrating that inhibition of renal phosphate reabsorption by PTH and FGF23 is NHERF1 dependent (12).
Current treatment of XLH includes supplementation with phosphate and 1,25D, or antibody-mediated inhibition of FGF23 activity (4, 13, 14). Studies in the Hyp mouse model of XLH demonstrate that daily 1,25D treatment without phosphate supplementation improves serum phosphate levels and the skeletal phenotype, despite a dramatic increase in serum FGF23 levels and bone FGF23 mRNA expression (15). 1,25D also increases expression of FAM20C, which phosphorylates FGF23, promoting its cleavage (15, 16).
Based on the significant decrease in urinary phosphate/urinary creatinine ratio and increased serum phosphate in Hyp mice treated daily with 1,25D, despite a dramatic increase in FGF23 expression, studies were undertaken to determine the molecular basis by which 1,25D impairs FGF23 signaling in Hyp mice.
Materials and Methods
Animal studies
Female and male Hyp mice in the C57BL/6J background were maintained in a virus- and parasite-free barrier facility and exposed to a 12-hour light/12-hour dark cycle. Mice were fed house chow (1% calcium, 0.6% phosphate) ad libitum, treated with 1,25D or vehicle from day 2 to 34, and euthanized on day 35 (15). Four-week old Hyp mice were injected subcutaneously with 1,25D (175 pg/g; Akron, Inc., Lake Forest, IL) or vehicle 1 hour or 18 hours before euthanasia. Wild-type (WT) mice were treated with PTH 1-34 (50 nmol/kg) or vehicle (17) 15 minutes after 1,25D treatment and euthanized 45 minutes later (1 hour after 1,25D treatment). All experimental procedures were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Biochemistry
Serum and urine were collected after 2 hours of fasting, and 1 or 18 hours after treatment with 1,25D or vehicle. Calcium and creatinine were measured using kits from Stanbio (Stanbio Laboratory, Boerne, TX); serum and urinary phosphate were measured using a kit from Abcam (catalog no. ab65622; Cambridge, MA). PTH was measured using the Mouse Intact PTH 1-84 Kit (60-2305; Immutopics, San Clemente, CA). C-terminal FGF23 (15) and Intact FGF23 were quantitated using the Mouse C-Terminal FGF23 Kit (catalog no. 60-6300; Immutopics) and Mouse Intact-FGF23 Kit (catalog no. 60-6300; Immutopics), respectively.
Isolation of renal brush-border membranes
Renal brush-border membranes (BBMs) were prepared by double magnesium chloride (MgCl2) precipitation as previously described (18) with minor modifications. Freshly isolated kidneys were washed in cold PBS supplemented with 0.1 mM phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich, St. Louis, MO). After removal of the renal capsule, the kidney was bisected coronally to permit removal of the renal hilum and pelvis, major calyces, and part of the inner medulla. The remaining tissue, enriched in renal cortex, was homogenized in 2 mL of cold 2x homogenization buffer (12 mM Tris pH 7.4, 300 mM mannitol, 5 mM EGTA). MgCl2 was added to a final concentration of 12 mM and samples were incubated on ice for 15 minutes with occasional mixing. Aggregated membranes were removed by 15-minute centrifugation at 3,000g, 4°C, and the supernatant was then centrifuged for 30 minutes at 40,000g, 4°C. The pellet was resuspended in 1 mL of 1x cold homogenization buffer supplemented with 12 mM MgCl2. After a second incubation and 15-minute centrifugation at 3,000g, 4°C, the supernatant was recovered and centrifuged at 40,000g, 4°C, for 30 minutes. The BBM pellets were resuspended in immunoprecipitation buffer (50 mM Tris pH7.4, 5 mM EDTA, 150 mM NaCl). All solutions were supplemented with phosphatase and protease inhibitors [2 mM β-glycerophosphate, 1 mM sodium orthovanadate (NaV), 10 mM sodium fluoride, and protease inhibitor cocktail (PIC Abcam-5621)].
Renal proximal tubule isolation
Renal cortical tissue was minced and washed with PBS supplemented with 1 mM PMSF. The minced cortices were incubated in a 37°C shaker at 100 rpm for 15 minutes in a PBS-collagenase solution (collagenase type II, 2 mg/mL, 5 mM glucose, 1 mg/mL BSA, 0.1 mg/mL DNase, 1 mM heptanoic acid, 1 mM PMSF, 1 mM NaV). The resultant tubules were washed in cold PBS supplemented with 5 mM glucose, protease inhibitors, and 10% heat-inactivated fetal bovine serum. After centrifugation at 35,000g for 30 minutes, the pellet was resuspended in cold 45% Percoll (Sigma-Aldrich) in HEPES pH7.4 and centrifuged for 35 minutes at 35,000g, 4°C. The resultant tubular pellet was washed once in PBS.
In vitro treatment of renal tubules
Renal tubules were isolated from the cortex by digestion with 0.25% trypsin (Gibco, BRL, New York) as described (19). The tubules from each animal were divided in half and incubated at 37°C 5% carbon dioxide for 1 hour in DMEM 10% fetal bovine serum supplemented with 10−7 M 1,25D, or vehicle. BBMs were then isolated for Western analyses.
Cell culture
Human renal proximal tubule epithelial cells immortalized with hTERT (RPTEC) were obtained from ATCC under a license from Geron Corp. Cells were cultured in DMEM/F-12 (10-090-CV; Corning) supplemented with 5 pM triiodo-l-thyronine, 10 ng/mL recombinant human epidermal growth factor, 25 ng/mL prostaglandin E1, 3.5 μg/mL ascorbic acid, 1 mg/mL insulin, 0.55 mg/mL transferrin, 0.5 μg/mL sodium selenite, 25 ng/mL hydrocortisone (catalog no. 740551; Millipore-Sigma, St. Louis, MO) plus 1% penicillin and streptomycin and 0.1 mg/mL G418. Cells were seeded on 6-well plates. After 72 hours, 3 wells of cells were treated for 1 hour with ethanol vehicle, 10 nM 1,25-dihydroxyvitamin D3 (catalog no. 740551; Millipore-Sigma) and/or 100 nM recombinant human R179Q-FGF23 (25–251), which is resistant to furin cleavage and inactivation (catalog no. 2604-FG-025; Fisher Scientific, Waltham, MA).
Protein extraction, SDS-PAGE, and Western blotting
Humeri from mice treated with 1,25D (175 pg/g) or vehicle from day 2 to day 34 were dissected free of muscle and connective tissue after which both growth plates were removed, and marrow was flushed with PBS to permit isolation of bone. The bone was homogenized in lysis buffer (catalog no. 9803; Cell Signaling, Danvers, MA) supplemented with 0.1 mM 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride (ESBF; Thermo Fisher Scientific, Waltham, MA), and protease inhibitor cocktail (catalog no. 5621; Abcam, Cambridge, MA). Proteins were subjected to immunoprecipitation to detect intact FGF23 and cleavage products.
Proteins from renal cortex, PTCs, and BBMs were lysed in radioimmunoprecipitation assay buffer (25 mM Tris-HCl, 150 mM NaCl, 1% TritonX100) supplemented with protease and phosphatase inhibitors (2 mM β-glycerophosphate, 1 mM NaV, 10 mM sodium fluoride, and PIC-Abcam 5621). Renal cortex (10 μg), PTC, and BBMs (20 μg) were subjected to SDS-PAGE and transferred to nitrocellulose membrane (Amersham Protran 0.45 μm, GE Healthcare, Germany). After incubation with the primary antibodies [NaPi2a; 2 μg/mL (catalog no. NBP2-42216, lot A2; Novus Biologicals, Centennial, CO)], NHERF1 (20) (4 μg/mL), phospho ERK1/2 (21) (0.18 μg/mL), total ERK (22) (0.01 μg/mL), and alkaline-phosphatase (23) (0.5 μg/mL) proteins were detected with horseradish peroxidase-conjugated secondary antibodies and the ECL system (PerkinElmer, Waltham, MA) or Super Signal West Femto maximum sensitivity substrate (Thermo Fisher Scientific).
Immunoprecipitation
FGF23 was immunoprecipitated from lysates of d35 humeral diaphyses of Hyp mice treated daily with 1,25D from day 2 to day 34 and WT vehicle-treated and Hyp mice. Protein lysates (200 μg) were precleared with Protein A/G Ultra Link beads (catalog no. 53132; Thermo Fisher Scientific) for 2 hours and immunoprecipitated using FGF23 antibodies (catalog no. 186-206, lot JSO20915-P1 and 225-224 lot JSO20915-P2; Immutopics, San Diego, CA). The immunoprecipitated proteins (12% of the total immunoprecipitate, approximately 25 μg starting material) were subject to Western analyses using antibodies against intact FGF23 (1 μg/mL) (24) and C-terminal-FGF23 (1 μg/mL; catalog no. 186-206, lot JSO20915-P1; Immutopics). Renal NHERF1 was immunoprecipitated in a similar fashion from renal cortical lysates using anti-NHERF1 (25, 26).
Quantitative RT-PCR
Total RNA was isolated from renal cortex and proximal tubular cells using the RNeasy Mini Kit (Thermo Fisher Scientific). RNA was reverse transcribed with Prime Script (Takara-Clontech, Shiga, Japan) after which quantitative RT-PCR was performed using the QuantiTect SYBR Green RT‐PCR Kit (Qiagen, Germany) on a Bio-Rad Real-time PCR (RT PCR2, Bio-Rad, CA). Gene expression was normalized to actin expression in each sample, using the method of Livak and Schmittgen (27). The primers sets utilized were: PTH1R (F, GCCATTGAGAACGAAACCAT; R, GTTTCCCATTCTTCCTGCAA), ACTIN (F, CCTCTATGCCAACACAGTGC; R, ACATCTGCTGGAAGGTGGAC), EGR1 (F, AGCGCCTTCAATCCTCAAG; R, CCACCATCGCCTTCTCATTAT), FGFR3 (F, CCTCGCTTCTGCGTTTGA; R, TTAGCACCCCTTGCAGCCTCT), FGFR4 (F, TCAAGGGACAAAGCTGGT; R, TCAAGGGACAAAGCTGGTG), FGFR1C (F, GGT-GGGCTTCTCTGTCATCATCTA; R,AGGGAGCTCATATTCGGAGA),αKLOTHO (F, GTACCTGGTTGCCCACAA; R, GGCAAACCAGCCTAGCA),NAPI2A (F, GGTCCAGTACCTCTACATC; R, CCCATGATGATCGGAAT).
Statistical analysis
All data are reported as the mean ± SEM or mean ± SD. One-way ANOVA followed by Fisher least significant difference was used to determine significance between groups. GraphPad Prism 7 was used for analyses; P < 0.05 was considered significant.
Results
1,25D does not alter FGF23 cleavage in the bone of Hyp mice
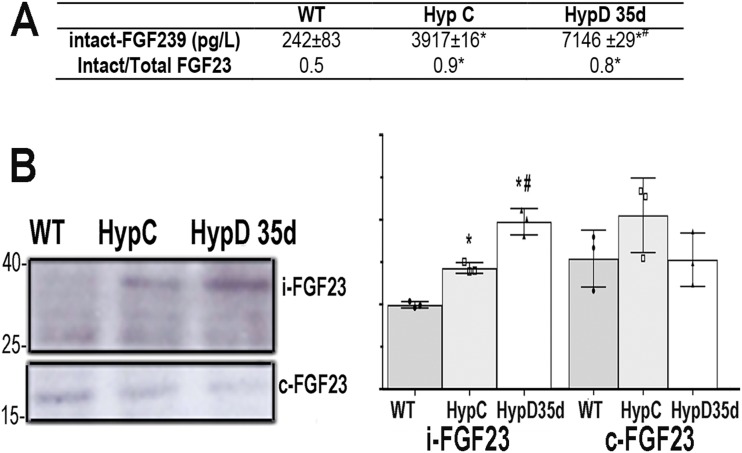
Daily 1,25D treatment of Hyp mice improves their mineral ion and skeletal phenotypes. This treatment results in a dramatic increase in circulating C-terminal FGF23 and enhanced mRNA expression of FAM20C, the protein that phosphorylates FGF23, promoting its cleavage (15). To determine whether 1,25D treatment impairs the phosphaturic effect of FGF23 by increasing its cleavage, cortical bone protein was isolated from the humeri of d35 Hyp mice treated with 1,25D from day 2 to day 34, and from vehicle-treated Hyp and WT mice. Circulating intact FGF23 levels were evaluated as well. An increase in intact circulating FGF23 was observed in the 1,25D-treated Hyp mice, but the ratio intact/total FGF23 in the circulation of these mice was not changed relative to that seen in vehicle-treated Hyp mice (Fig. 1A). Consistent with these findings, Western analyses demonstrate that the increase in cortical bone FGF23 protein in Hyp mice was further increased by 1,25D treatment, but no increase in FGF23 cleavage products was observed (Fig. 1B). Thus, improvement in renal phosphate handling in 1,25D-treated Hyp mice is not secondary to enhanced FGF23 cleavage.
Figure 1.
1,25D treatment does not alter FGF23 cleavage in Hyp mice. (A) Circulating intact FGF23 and intact/total FGF23 ratio. Data represent the mean ± SD of results from four mice per group. *P < 0.05 vs WT; #P < 0.05 vs Hyp control mice. (B) Immunoprecipitation of FGF23 from cortical bone. Intact (i-FGF23) and C-terminal (c-FGF23) FGF23 fragments in WT and Hyp mice treated or not with 1,25D. Quantitation of band intensity on Western analyses from three mice per genotype and treatment group. Hyp C, vehicle-treated Hyp mice; HypD35d, day 35 Hyp mice treated with 1,25D from day 2 to day 34; WT, vehicle-treated WT mice.
Acute 1,25D treatment attenuates urinary phosphate wasting in Hyp mice
Day 35 Hyp mice treated from day 2 to day 34 with 1,25D show a reduction in renal phosphate wasting compared with vehicle-injected Hyp mice (15). To determine whether these effects of 1,25D are seen acutely, biochemical analyses were performed at 1 and 18 hours post-1,25D or vehicle treatment of Hyp mice. As early as 1 hour, 1,25D significantly reduced the urinary phosphate/urinary creatinine ratio in Hyp mice. An increase in serum phosphate levels accompanied this decrease in renal phosphate wasting. These changes persisted 18 hours after a single injection of 1,25D (Table 1).
Table 1.
Serum and Urine Biochemistry
| WT | Hyp C | HypD 1h | HypD 18h | |
|---|---|---|---|---|
| Pi, mg/dL | 9.53 ± 0.89 | 5.99 ± 0.81a | 7.2 ± 0.52a,b | 7.48 ± 0.75a,b |
| uPi/uCr | 3.59 ± 1.43 | 5.96 ± 1.7 a | 4.22 ± 1.25b | 4.05 ± 1.8b |
| Ca, mg/dL | 8.61 ± 1.41 | 8.98 ± 1.14 | 8.78 ± 1.33 | 8.55 ± 1.02 |
Data in mean ± SD is representative of that obtained from 6 to 15 mice per group.
Abbreviations: Ca, serum calcium; Hyp C, vehicle-treated Hyp mice; HypD1h, Hyp mice treated with 1,25D for 1 h; HypD18h, Hyp mice treated with 1,25D for 18 h; Pi, serum phosphate; uCr, urinary creatinine. uPi, urinary phosphate; WT, vehicle-treated WT mice.
P < 0.05 vs WT.
P < 0.05 vs Hyp control mice.
1,25D does not impair FGF23 receptor expression or signaling in the kidneys of Hyp mice
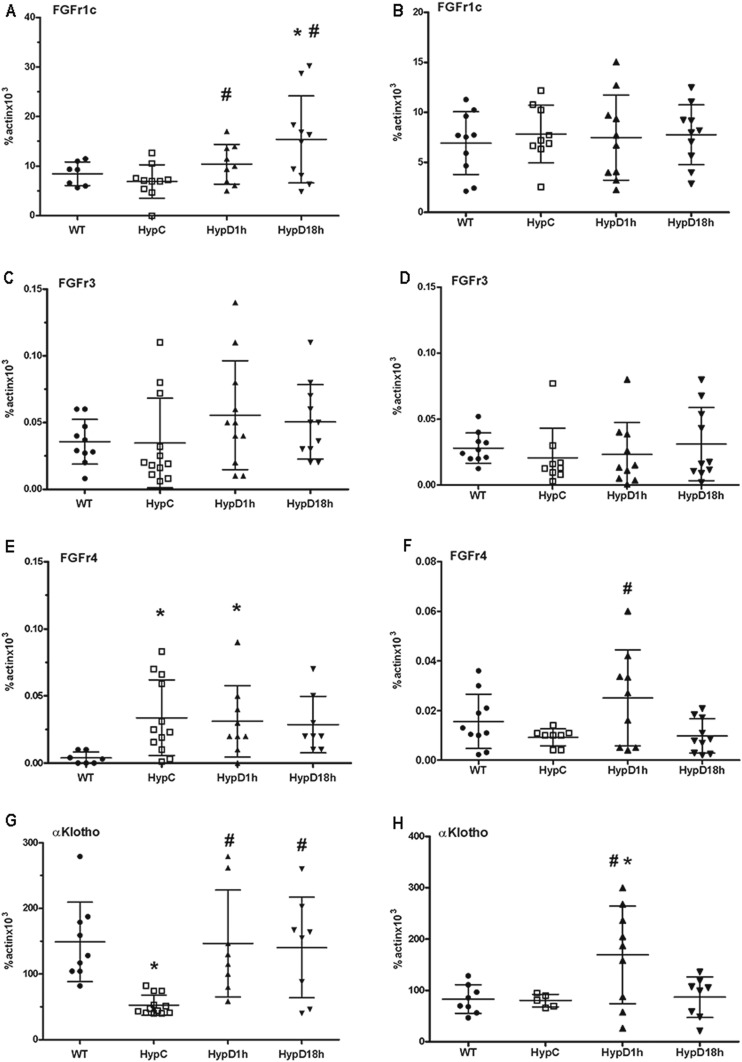
Because treatment of Hyp mice with 1,25D improves renal phosphate wasting within 1 hour, studies were performed to determine whether 1,25D acutely impairs FGF23 receptor expression or signaling in Hyp mice. Thus, mRNA expression of FGF receptors and the FGF23 coreceptor α-Klotho was examined in the renal cortex and PTCs isolated from WT and 1,25D- or vehicle-treated Hyp mice. The expression of FGFR1c, the principal FGF23 receptor involved in renal phosphate handling, was similar in the renal cortex and PTCs of WT and Hyp control mice. FGFR1c mRNA expression in the renal cortex, but not in PTCs was increased 1 and 18 hours after 1,25D injection into Hyp mice, suggesting this induction was in non-PTC regions of the kidney (Fig. 2A and 2B). The expression of FGFR3 mRNA in the renal cortex and PCT did not differ between the vehicle-treated WT and Hyp mice, and was not altered by 1,25D (Fig. 2C and 2D). FGFR4 mRNA expression was increased in the renal cortex of Hyp control vs WT mice and was increased in the PTCs of Hyp mice 1 hour after injection of 1,25D (Fig. 2E and 2F). The expression of the FGF23 coreceptor α-klotho was reduced in the renal cortex of Hyp control mice relative to that seen in WT mice but was normalized after 1 and 18 hours of 1,25D treatment. PTC mRNA expression of α-klotho was similar between WT and Hyp control mice (Fig. 2G and 2H). As was seen for FGFR4, 1 hour of 1,25D treatment increased α-klotho mRNA expression in the PTC's of Hyp mice. Cortical α-klotho expression was also increased by 1,25D.
Figure 2.
1,25D treatment does not inhibit renal FGF receptor or coreceptor expression. mRNA expression of (A, C, E, G) FGF receptors 1c, 3, 4, and α-Klotho in renal cortex and (B, D, F, H) PTCs of vehicle-treated WT mice. Data represent the mean ± SEM. *P < 0.05 vs WT; #P < 0.05 vs Hyp control mice. Hyp C, vehicle-treated Hyp mice; HypD1h, Hyp mice treated with 1,25D for 1 h; HypD18h, Hyp mice treated with 1,25D for 18 h; WT, vehicle-treated WT mice.
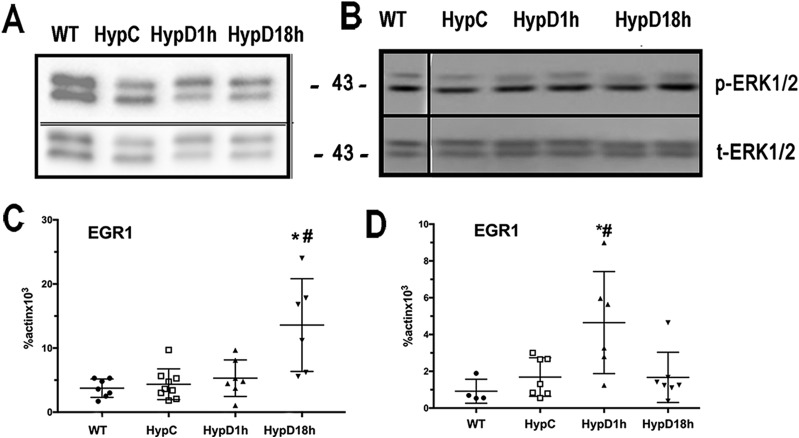
FGF23-FGF receptor interactions lead to activation of the MAPK signaling pathway and induction of gene expression (8). To address whether downstream signaling by FGF receptors is impaired by 1,25D treatment of Hyp mice, the effect of 1,25D on Erk1/2 phosphorylation and mRNA expression of the early response gene, EGR1, was examined in renal cortical and PTC lysates. 1,25D did not alter the phosphoERK1/2/total ERK1/2 ratio in the renal cortex or PTCs of Hyp mice, suggesting that 1,25D does not attenuate renal phosphate wasting by impairing activation of the MAPK signaling pathway (Fig. 3A and 3B). Consistent with this, EGR1 mRNA was not suppressed but was actually induced by 1,25D in both the renal cortex and PTCs of Hyp mice (Fig. 3C and 3D).
Figure 3.
1,25D does not suppress FGF23 signaling in Hyp renal cortex or proximal tubular cells. Western analyses of phospho ERK1/2 (p-ERK1/2) and total ERK1/2 (t-ERK1/2) in (A) renal cortex and (B) proximal tubular cells. The migration of the 43-kDa marker is indicated. Data are representative of that obtained from three mice per group. EGR1 mRNA expression in (C) renal cortex and in (D) proximal tubular cells. Data represents the mean ± SEM. *P < 0.05 vs WT; #P < 0.05 vs Hyp control mice. Hyp C, vehicle-treated Hyp mice; HypD1h, Hyp mice treated with 1,25D for 1 h; HypD18h, Hyp mice treated with 1,25D for 18 h; WT, vehicle-treated WT mice.
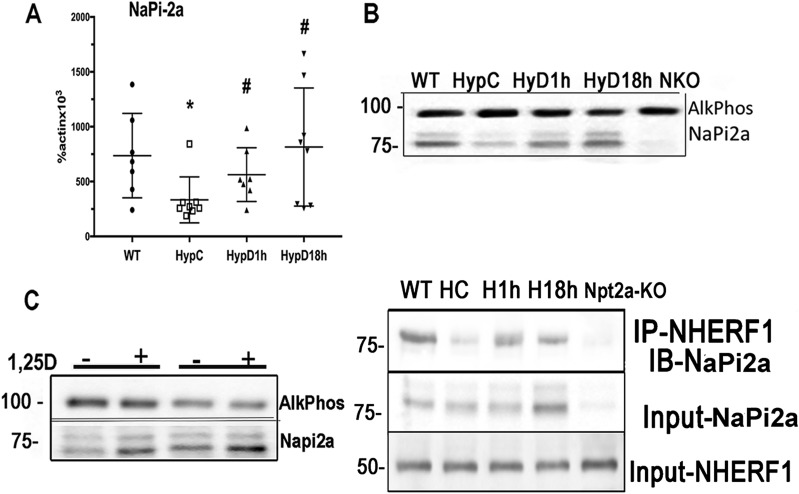
1,25D increases NaPi2a mRNA levels and BBM NaPi2a protein localization in Hyp mice
The sodium phosphate cotransporter NaPi2a is the major regulator of renal phosphate reabsorption (28). BBM localization of this protein is critical for its effects on renal phosphate transport. Because 1,25D treatment improves renal phosphate wasting in Hyp mice, without decreasing FGFR expression or impairing MAPK signaling in PTCs, studies were performed to determine its effects on NaPi2a mRNA expression and NaPi2a protein localization. NaPi2a mRNA expression was reduced in Hyp control mice compared with WT mice. 1,25D normalized NaPi2a mRNA expression in Hyp mice after 1 and 18 hours (Fig. 4A). 1,25D markedly enhanced BBM NaPi2a protein expression in the Hyp mice by 1 hour after 1,25D injection. This enhanced BBM NaPi2a was sustained for 18 hours (Fig. 4B). To determine whether these findings could be recapitulated in vitro, RPTEC cells were treated with 1,25D for 1 hour prior to FGF23 treatment. Unlike what was observed in vivo, 1,25D did not attenuate the dramatic decrease in Napi2a/NHERF1 interactions seen in response to FGF23 (data not shown). Because these RPTEC cells were not polarized, and thus do not have the same cytoskeletal organization as PTCs, studies were performed with proximal tubules isolated from Hyp mice. Proximal tubules isolated from each mouse were divided in half and cultured in the presence of 1,25D or vehicle for 1 hour, after which BBMs were isolated and subject to Western analyses. In vitro treatment of these tubules was able to recapitulate the increase in BBM NaPi2a observed with in vivo treatment of Hyp mice (Fig. 4C).
Figure 4.
1,25D promotes BBM NaPi2a localization. (A) NaPi2a mRNA expression. Data represent the mean ± SEM. *P < 0.05 vs WT; #P < 0.05 vs Hyp control mice. (B) NaPi2a protein expression in renal BBMs from Hyp mice treated or not with 1,25D. The migration of the 100-kDa [alkaline phosphatase (AlkPhos) control] and 75-kDa markers is indicated. (C) NaPi2a protein expression in BBM of Hyp renal tubules treated in vitro with 1,25D or vehicle for 1 h. Alkaline phosphatase (AlkPhos) is used as control. Data are representative of that obtained from four Hyp mice; data from one male and one female Hyp mouse are shown. (D) Immunoprecipitation of NaPi2a from renal cortical lysates with anti-NHERF1. NaPi2a and NHERF1 protein input. The migration of the 75- and 50-kDa markers is indicated. Data are representative of that obtained from three mice per group. Hyp C, vehicle-treated Hyp mice; HypD1h, Hyp mice treated with 1,25D for 1 h; HypD18h, Hyp mice treated with 1,25D for 18 h; Npt-KO, Npt2 knockout; WT, vehicle-treated WT mice.
BBM localization of NaPi2a is regulated by its interactions with the scaffold protein NHERF1 (11). To address whether the enhancement of renal BBM NaPi2a seen in 1,25D-treated mice is associated with enhanced NaPi2a/NHERF1 interactions, immunoprecipitations were performed using renal cortical lysates from Hyp mice treated with 1,25D or vehicle for 1 or 18 hours. NHERF1 immunoprecipitation analyses demonstrated impaired NaPi2a-NHERF1 interactions in vehicle-treated Hyp mice compared with WT mice. Treatment with 1,25D enhanced NaPi2a-NHERF1 interactions as early as 1 hour and this persisted for 18 hours. An increase in NaPi2a protein in the cortical lysates of Hyp mice was observed 18 hours post-1,25D treatment, but not 1-hour posttreatment. Neither NHERF1 protein (Fig. 4D) nor mRNA levels (data not shown) were altered by 1,25D treatment.
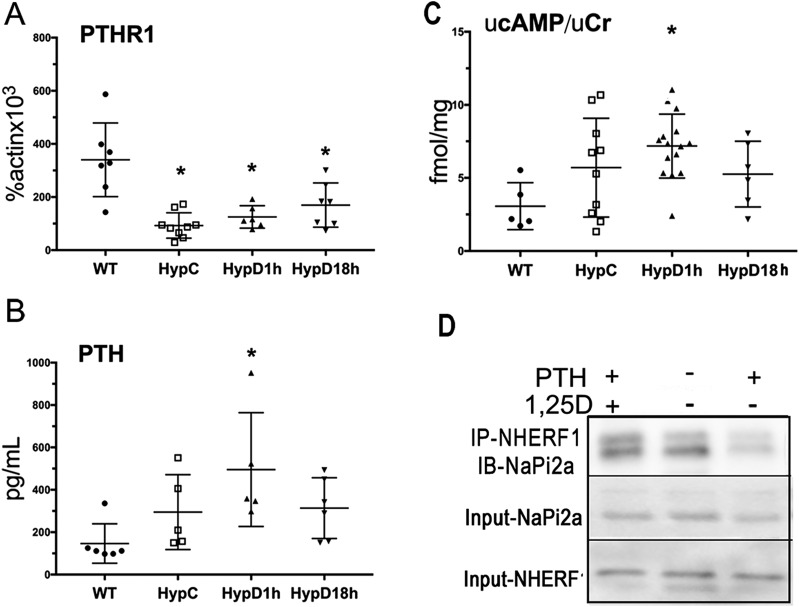
Acute 1,25D treatment does not impair PTH signaling
PTH and FGF23 regulate renal PTC phosphate transport. Because 1,25D has been shown to suppress PTH1R mRNA expression in osteosarcoma cells (29), studies were performed to address whether 1,25D attenuates renal phosphate wasting in Hyp mice by decreasing PTH receptor levels, decreasing circulating PTH, or impairing cAMP generation. The expression of the PTH1R was suppressed in the PTCs of Hyp mice, relative to WT mice, but was not further suppressed by 1,25D treatment (Fig. 5A). A paradoxical increase in circulating PTH levels, relative to WT mice, was seen 1 hour after 1,25D injection of Hyp mice (Fig. 5B). Activation of adenylyl cyclase disrupts NaPi2a/NHERF1 interactions (30, 31), leading to decreased BBM NaPi2a and renal phosphate wasting. Consistent with the increase in circulating PTH levels seen 1 hour after 1,25D administration, an increase in urinary cAMP/creatinine ratio was observed in Hyp mice (vs WT controls), demonstrating that 1,25D does not acutely impair PTC PTH signaling (Fig. 5C). Although these data are consistent with the findings that the effects of 1,25D on BBM NaPi2a in Hyp mice are not the result of systemic effects (Fig. 4C), they raise the question as to whether 1,25D also causes PTH resistance. To address this, WT mice were treated with PTH 15 minutes after 1,25D and euthanized 45 minutes later (1 hour after 1,25D treatment). Although PTH treatment resulted in a significant increase in renal phosphate excretion [control urinary phosphase (uPi)/urinary creatinine (uCr) 4.3 ± 2.1 vs PTH alone 8.8 ± 2.2, P = 0.05), 1,25 D pretreatment attenuates this effect (uPi/uCr = 6.1 ± 3.7, P = 0.5 vs control, P = 0.09 vs PTH alone). To address if this effect of 1,25D on PTH-mediated renal phosphate wasting was associated with preservation of NaPi2a/NHERF1 interactions, NHERF1 was immunoprecipitated from the renal cortex of these mice and immunoblotted for NaPi2a. Although neither NHERF1 nor NaPi2a protein levels were affected by PTH or 1,25D, treatment with 1,25D prevented the decrease in NHERF1/NaPi2a interactions observed with PTH alone (Fig. 5D).
Figure 5.
Acute 1,25D treatment does not impair PTH signaling. (A) PTHR1 mRNA expression in proximal tubular cells. (B) Circulating PTH levels. (C) Urinary cAMP/creatinine ratio. Data represent the mean ± SEM. *P < 0.05 vs WT; #P < 0.05 vs Hyp control mice. Vehicle-treated wild-type mice (WT), vehicle-treated Hyp mice (HypC), and Hyp mice treated with 1,25D for 1 (HypD1h) or 18 (HypD18h) h. (D) Immunoprecipitation of NaPi2a with anti-NHERF1 from renal cortical lysates of WT mice treated with vehicle, PTH alone, or PTH and 1,25D. NaPi2a and NHERF1 protein input. Data are representative of that obtained from three independent replicates per treatment group.
Discussion
These investigations were undertaken to identify the molecular basis for the improvement in renal phosphate wasting in 1,25D-treated Hyp mice. Although our previous investigations were performed in mice treated with 1,25D on a daily basis from day 2 to day 34 or 74 of life, the current studies demonstrate that the beneficial effect of 1,25D on renal phosphate handling in Hyp mice is seen as early as 1 hour postadministration of 1,25D and is maintained for at least 18 hours. The current studies demonstrate that this improvement in renal phosphate handling is paradoxically accompanied by enhanced FGF23 and PTH signaling, evidenced by an increase in EGR1 mRNA expression and urinary cAMP/creatinine ratio, as early as 1-hour postadministration of 1,25D. Despite this, and consistent with the decrease in urinary phosphorus/creatinine ratio, 1,25D enhances renal brush border NaPi2a protein levels within 1 hour in Hyp mice.
Reabsorption of phosphate by the proximal renal tubule requires expression of specific transporters at the luminal membrane. NaPi2a is the primary regulator of proximal renal tubule phosphate transport, as evidenced by the phenotype of mice (2, 32) and humans (33) with inactivating mutations of this gene. The two major phosphate-regulating hormones, PTH and FGF23, exert their phosphaturic effects by decreasing the amount of NaPi2a protein at the proximal tubule brush border. Mutational analyses demonstrate that the three carboxy-terminal amino acids of NaPi2a are required for interactions with the scaffolding protein NHERF1, and its brush border localization (34).
NHERF1 contains two PDZ domains (35), the first of which is both necessary and sufficient for NHERF1-NaPi2a interactions (10, 36). Consistent with the role of NHERF1 in maintaining NaPi2a at the brush border, NHERF1-KO mice exhibit renal phosphate wasting associated with decreased BBM NaPi2a (11). Of note, the absence of NHERF1 abolishes both PTH and FGF23 regulation of NaPi2a brush border localization and renal phosphate transport (12, 37).
Phosphorylation of NHERF1Ser77 in response to PTH activation of cAMP impairs NaPi2a/NHERF1 interactions, and thus, renal proximal tubule phosphate transport (12, 37, 38). FGF23 inhibition of phosphate transport is also NHERF1pSer77 dependent, but FGF23 is believed to promote this phosphorylation by activation of pERK1/2: pSGK1. Consistent with this, inhibitors of PKC, PKA, and pERK1/2 inhibit the effects of PTH on phosphate transport in cultured cells, whereas inhibitors of pSGK1 and pERK1/2 inhibit the effects of FGF23 (39). Although our studies demonstrate that 1,25D treatment did not alter pERK1/2 in the PTCs of Hyp mice, we were unable to detect pSGK1 in the PTC lysates. pSGK1 levels were not altered in the kidney lysates of 1,25D-treated Hyp mice relative to vehicle-treated mice (data not shown), consistent with the high level and FGF23-independent expression of this mineralocorticoid responsive kinase in renal distal tubules (40).
The effect of 1,25D on renal phosphate reabsorption is less well understood. Previous studies in 1,25D-treated Hyp mice reveal a significant improvement in renal phosphate handling and skeletal phenotype despite a further increase in circulating FGF23 levels (15). Long-term treatment of Hyp mice with the vitamin D analogue, eldecalcitol, also increases NaPi2a protein levels in renal BBMs, accompanied by an increase in NHERF1 protein levels (41). Our investigations demonstrate that, by one hour after administration of a single dose of 1,25D, NaPi2a increases in proximal tubule BBMs and NaPi2a/NHERF1 interactions are enhanced. Both of these occur without an increase in the level of either NaPi2a or NHERF1 protein levels. These rapid effects that occur without a change in protein expression suggest that 1,25D inhibits FGF23 and/or PTH signaling. However, 1,25D acutely increases αklotho and FGFr4 mRNA expression in the PTCs of Hyp mice. Furthermore, 1,25D increases mRNA expression of EGR1, which is required for FGF23-mediated inhibition of renal phosphate transport (9). Similarly, although 1,25D has been shown to suppress PTH levels and expression of its receptor, PTH1R mRNA expression was not altered, whereas a paradoxical increase in circulating PTH and urinary cAMP/creatinine, an indicator of PTH activity, is observed one hour after 1,25D administration to Hyp mice, suggesting that 1,25D causes PTH resistance as well. Consistent with this, 1,25D is able to decrease PTH-dependent phosphaturia and maintain NHERF1/NaPi2a interactions in PTH-treated WT mice.
Although these studies clearly demonstrate that 1,25D improves renal phosphate transport in Hyp mice by maintaining NaPi2a at the proximal tubule brush border without impairing PTH or FGF23 receptor expression or downstream signaling, they raise questions regarding potential mechanisms involved. Because 1,25D exerts its effect in isolated renal tubules and increases serum phosphate and decreases urinary phosphate wasting within one hour in fasting Hyp mice, enhancement of intestinal phosphate transport or paracellular phosphate transport cannot be implicated. In addition, 1,25D has no effects on renal phosphate transport in WT mice, supporting its role in antagonizing the effects of PTH and FGF23 on NaPi2a/NHERF1 interactions. Consistent with this, the lack of effect of 1,25D on urinary phosphate excretion in NaPi2a knockout mice (urine phosphate/creatinine 5.4 ± 2.6 vs untreated 4.6 ± 2.3, P = 0.6) supports the critical role of NaPi2a. The inability of 1,25D to maintain NaPi2a/NHERF1 interactions in FGF23 treated nonpolarized RPTEC cells suggests the cytoskeletal organization of the proximal tubule is required for the effects of 1,25D.
Taken together, these studies demonstrate that 1,25D regulates hormone-dependent interactions of the NaPi2a-NHERF1 complex at the proximal tubule luminal brush border that relies on precise cellular polarization and a preserved cellular cytoskeletal organization that cannot be recapitulated in cultured cell models. Future investigations will be required to determine whether the effects of 1,25D reflect novel posttranslational modifications that enhance NaPi2a/NHERF1 interactions, specific antagonism of PTH and FGF23 actions, or involvement of other scaffolding/ cytoskeletal proteins that stabilize NaPi2a at the proximal tubule brush border.
Acknowledgment
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Support: This work was supported by grants from the National Institutes of Health (R01 R01-AR072650 to M.B.D.; K08-AR067854 to E.S.L.; and R01-DK105811, R01-DK111427 to P.A.F.).
Glossary
Abbreviations:
- 1,25D
1,25-dihydroxyvitamin D
- BBM
brush border membrane
- EGR1
early growth response 1
- FGF
fibroblast growth factor
- FGF23
fibroblast growth factor 23
- MgCl2
magnesium chloride
- NaV
sodium orthovanadate
- NHERF1
Na-H+ exchange regulatory factor 1
- PMSF
phenylmethylsulfonyl fluoride
- PTC
proximal tubular cell
- XLH
X-linked hypophosphatemia
- WT
wild-type
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. [DOI] [PubMed] [Google Scholar]
- 2. Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol. 2003;285(6):F1271–F1278. [DOI] [PubMed] [Google Scholar]
- 3. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. [DOI] [PubMed] [Google Scholar]
- 4. Kinoshita Y, Fukumoto S. X-linked hypophosphatemia and FGF23-related hypophosphatemic diseases: prospect for new treatment. Endocr Rev. 2018;39(3):274–291. [DOI] [PubMed] [Google Scholar]
- 5. Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, Xie Y, Drezner MK. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118(2):722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297(2):F282–F291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab. 2011;300(3):E508–E517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farrow EG, Summers LJ, Schiavi SC, McCormick JA, Ellison DH, White KE. Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the Hyp mutation. J Endocrinol. 2010;207(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Portale AA, Zhang MY, David V, Martin A, Jiao Y, Gu W, Perwad F. Characterization of FGF23-dependent Egr-1 cistrome in the mouse renal proximal tubule. PLoS One. 2015;10(11):e0142924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernando N, Déliot N, Gisler SM, Lederer E, Weinman EJ, Biber J, Murer H. PDZ-domain interactions and apical expression of type IIa Na/P(i) cotransporters. Proc Natl Acad Sci USA. 2002;99(18):11957–11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA. 2002;99(17):11470–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weinman EJ, Steplock D, Shenolikar S, Biswas R. Fibroblast growth factor-23-mediated inhibition of renal phosphate transport in mice requires sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) and synergizes with parathyroid hormone. J Biol Chem. 2011;286(43):37216–37221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Insogna KL, Briot K, Imel EA, et al. . A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1383–1393. [DOI] [PubMed] [Google Scholar]
- 14. Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu ES, Martins JS, Raimann A, Chae BT, Brooks DJ, Jorgetti V, Bouxsein ML, Demay MB. 1,25-Dihydroxyvitamin D alone improves skeletal growth, microarchitecture, and strength in a murine model of XLH, despite enhanced FGF23 expression. J Bone Miner Res. 2016;31(5):929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA. 2014;111(15):5520–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maeda A, Okazaki M, Baron DM, Dean T, Khatri A, Mahon M, Segawa H, Abou-Samra AB, Jüppner H, Bloch KD, Potts JT Jr, Gardella TJ. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci USA. 2013;110(15):5864–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loffing J, Lötscher M, Kaissling B, Biber J, Murer H, Seikaly M, Alpern RJ, Levi M, Baum M, Moe OW. Renal Na/H exchanger NHE-3 and Na-PO4 cotransporter NaPi-2 protein expression in glucocorticoid excess and deficient states. J Am Soc Nephrol. 1998;9(9):1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112(10):1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. RRID:AB_10990289, http://scicrunch.org/resolver/AB_10990289.
- 21. RRID:AB_331646, http://scicrunch.org/resolver/AB_331646.
- 22. RRID:AB_330744, http://scicrunch.org/resolver/AB_330744.
- 23. RRID:AB_664062, http://scicrunch.org/resolver/AB_664062.
- 24. RRID:AB_1964581, http://scicrunch.org/resolver/AB_1964581.
- 25. RRID:AB_307312, http://scicrunch.org/resolver/AB_307312.
- 26. RRID:AB_303814, http://scicrunch.org/resolver/AB_303814.
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 28. Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA. 1998;95(9):5372–5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie LY, Leung A, Segre GV, Yamamoto I, Abou-Samra AB. Downregulation of the PTH/PTHrP receptor by vitamin D3 in the osteoblast-like ROS 17/2.8 cells. Am J Physiol. 1996;270(4 Pt 1):E654–E660. [DOI] [PubMed] [Google Scholar]
- 30. Nagai S, Okazaki M, Segawa H, Bergwitz C, Dean T, Potts JT Jr, Mahon MJ, Gardella TJ, Jüppner H. Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem. 2011;286(2):1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang B, Means CK, Yang Y, Mamonova T, Bisello A, Altschuler DL, Scott JD, Friedman PA. Ezrin-anchored protein kinase A coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport. J Biol Chem. 2012;287(29):24148–24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tenenhouse HS, Beck L. Renal Na(+)-phosphate cotransporter gene expression in X-linked Hyp and Gy mice. Kidney Int. 1996;49(4):1027–1032. [DOI] [PubMed] [Google Scholar]
- 33. Prié D, Huart V, Bakouh N, Planelles G, Dellis O, Gérard B, Hulin P, Benqué-Blanchet F, Silve C, Grandchamp B, Friedlander G. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347(13):983–991. [DOI] [PubMed] [Google Scholar]
- 34. Déliot N, Hernando N, Horst-Liu Z, Gisler SM, Capuano P, Wagner CA, Bacic D, O’Brien S, Biber J, Murer H. Parathyroid hormone treatment induces dissociation of type IIa Na+-P(i) cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol. 2005;289(1):C159–C167. [DOI] [PubMed] [Google Scholar]
- 35. Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139(1):169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H. Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J Biol Chem. 2001;276(12):9206–9213. [DOI] [PubMed] [Google Scholar]
- 37. Weinman EJ, Biswas RS, Peng G, Shen L, Turner CL, E X, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1 [published correction appears in J Clin Invest. 2008;118(1):387.]. J Clin Invest. 2007;117(11): 3412–3420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol. 2012;303(3):F321–F327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sneddon WB, Ruiz GW, Gallo LI, Xiao K, Zhang Q, Rbaibi Y, Weisz OA, Apodaca GL, Friedman PA. Convergent signaling pathways regulate parathyroid hormone and fibroblast growth factor-23 action on NPT2A-mediated phosphate transport. J Biol Chem. 2016;291(36):18632–18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA. 1999;96(5):2514–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaneko I, Segawa H, Ikuta K, Hanazaki A, Fujii T, Tatsumi S, Kido S, Hasegawa T, Amizuka N, Saito H, Miyamoto KI. Eldecalcitol causes FGF23 resistance for Pi reabsorption and improves rachitic bone phenotypes in the male Hyp mouse. Endocrinology. 2018;159(7):2741–2758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.