Abstract
Background
Neonatal sepsis (NS) is a common systemic disease that causes morbidity and mortality in newborns. But there is no ideal biomarker that can be used in the early diagnosis of NS. In recent studies, platelet to lymphocyte ratio (PLR) has been reported to play a critical role in the inflammatory process. In this study, we aimed to contribute to the research about whether or not PLR can be used as an early predictor of the diagnosis of NS.
Methods
This retrospective cohort study was conducted among the newborns born in İzmir Buca Maternity and Pediatric Hospital between March 2015–February 2016. During these twelve months, 611 neonates with Early-Onset Sepsis (EOS) were admitted to our neonatal intensive care unit. One hundred and forty-nine neonates with suspected EOS, 67 neonates with proven EOS and 92 healthy neonates were enrolled in the study.
Results
Platelet to lymphocyte ratio (PLR) values of the three groups were calculated 56.5 ± 17.8 vs. 62.4± 14.9 vs. 15.3 ± 2.1, respectively. PLR values of suspected or proven EOS group were significantly higher than the control group. PLR has AUC 0.89 to 0.93, the cutoff value of 39.5 to 57.7, the sensitivity of 88.9% to 91.3% and specificity of 94.7% to 97.6%, the positive predictive value of 94.3% to 97.4%, and negative predictive value of 88.6% to 91.8% in suspected and proven sepsis diagnosis.
Conclusions
Our results suggest that PLR can be used as a parameter in the prediction of neonatal sepsis.
Keywords: PLR, Biomarker, Neonatal sepsis
Introduction
Neonatal sepsis (NS) is one of the major causes of morbidity and mortality in neonatal age.1 NS are classified, according to the absence or the presence of the positive blood culture, in Clinical Sepsis and Proven Sepsis. Concerning the time of symptoms onset, they are defined as Early-Onset Sepsis (EOS) and Late-Onset Sepsis (LOS). When the blood culture is negative, but the neonate presents clinical and inflammation signs, and biomarker increase, the sepsis is defined as Clinical Sepsis.
Conversely, in Proven Sepsis, the neonate presents clinical, and laboratory signs of infection/inflammation, and the blood cultures are positive.2 The time of onset defines the type of sepsis. The ones developing in the first three days of life are called EOS, whereas those developing from 4 to 28 days of life are called LOS.3 It is believed that EOS is mainly due to the maternal-fetal transmission of microorganisms during pregnancy or perinatally. Microorganism transmission to the blood circulation of neonates causes immune system reaction leading to systemic inflammatory response syndrome (SIRS), which may progress into sepsis, multiple organ failure, and death.4 Early diagnosis and therapy may inhibit the progression of SIRS and prevent sepsis-related morbidity and mortality.5 Determination of maternal risk factors and clinical and laboratory features are used for diagnosis of EOS. Important risk factors for EOS include the maternal medical history of urinary infection, vaginitis, early membrane rupture, and chorioamnionitis.6 Clinical signs are nonspecific and subtle in neonatal EOS. The unspecific clinical symptoms in neonates and the lack of sufficiently accurate biomarkers can lead to delay in diagnosis and initiation of the therapy, unnecessary hospital admissions, and antibiotic resistance secondary to antibiotic misuse.7 Blood culture is the gold standard laboratory test in the diagnosis of NS; however this method has significant limitations, which include false negativity secondary to maternal antibiotic use or low microorganism concentration, need 48 to 72 hours to get the results, false positivity secondary to contamination. Actually, the blood culture sensitivity in the diagnosis of sepsis is reported to be around 19%.8 Given that, a “magic” biomarker to early diagnose EOS is to find. Many biomarkers have been tested for the accuracy in EOS diagnosis, including acute phase reactants, interleukins, and immunoglobins.9–11 C-reactive protein (CRP) is the most frequently studied inflammatory marker, which is also used in the follow-up of therapy. CRP is a sensitive but not a specific marker to diagnose sepsis, because of the increase in multiple non-infectious inflammatory events, other than sepsis, and the delay in the increase (10 to 12 hours).12 Another inflammatory marker, procalcitonin (PCT), increases in the first 3 to 4 hours from the beginning of symptoms and decreases to normal level in 24 hours.13 Since peripheral blood smear test, another inflammatory marker, necessitates both appropriate laboratory conditions and personal experience, it’s reliability in sepsis diagnosis in low.14 All of these limitations regarding inflammatory markers cause the absence of a reliable biomarker that can be used in the early diagnosis of NS. Recent studies reported that platelet and lymphocytes have a critical role in the inflammatory process. PLR is an indicator of the balance between inflammation and thrombosis. Thus, the inflammatory status results in accelerated megakaryocyte proliferation and associated thrombocytosis. Moreover, increased platelet counts and decreased lymphocyte counts have been shown to be related to both aggregation and inflammation, and thus, represent risk indicators.15–18 In the present study, the PLR which are parts of a complete blood count, were compared with the traditional parameters CRP and PCT for the ability to predict EOS in neonates with or without positive blood cultures.
Materials and Methods
Patients
An observational, retrospective cohort study was conducted to evaluate newborns born in Buca Gynecology, Obstetrics and Pediatrics Hospital, Izmir, Turkey between March 2015 and February 2018. We calculated that a sample size of 64 in the study group and 64 in the control group would allow us to detect differences between the 2 groups (α = 0.05, power = 80%).19 Our patient group included neonates with the gestational age of 37 to 42 weeks according to Ballard Score or ultrasonography performed before week 20, appropriate for gestational age (AGA) and diagnosed with EOS. Exclusion criteria included less than 37 weeks or more than 42 weeks, small for gestational age (SGA), intrauterine growth restriction (IUGR), perinatal asphyxia, congenital abnormality, congenital heart disease, chromosomal abnormality, preeclampsia, and lack of data. Newborns with a maternal history of urinary tract infection, vaginitis, early membrane rupture, and clinical or histological chorioamnionitis in last trimester were followed up for 72 h for clinical signs related to sepsis, and sepsis screening was performed for newborns with clinical findings 12 h postnatally. Sepsis screening was performed for newborns without clinical signs related to sepsis at 12–24 h of the newborn with a maternal history of urinary tract infection, vaginitis, early membrane rupture, and clinical or histological chorioamnionitis in last trimester. Sepsis screening included a complete blood count (CBC), CRP, PCT, and peripheral blood smear. Lumbar puncture was performed for newborns with fever or seizure or neurological findings or positive blood culture. European Medicines Agency (EMA), Report on the Expert Meeting on Neonatal and Pediatric Sepsis criteria were used for the diagnosis of sepsis.20 Clinical signs were: (1) Respiratory instability: apnea, tachypnea, increased oxygen requirements, or requirement for ventilation support; (2) Cardiovascular instability: bradycardia, tachycardia, rhythm instability, reduced urinary output (less than 1 mL/kg/h), hypotension, mottled skin or impaired peripheral perfusion; (3) Modified body temperature: hypothermia, hyperthermia, or temperature instability; (4) Gastrointestinal instability: feeding intolerance, poor sucking, or abdominal distention; (5) Skin and subcutaneous lesions: petechial rash or sclerema; (6) Non-specific: irritability, lethargy, or hypotonia. Sepsis screening was performed at 12–24 h of life for these laboratory signs: (1) White blood cell (WBC) count < 4,000 × 109 cells/L or > 20,000 × 109 cells/L; (2) Immature to total neutrophil ratio (I/T) greater than 0.2; (3) Platelet count < 100,000 × 109 cells/L; (4) CRP >15 mg/L or PCT ≥ 2 ng/mL; (5) Glucose intolerance confirmed at least two times, hyperglycemia (blood glucose > 180 mg/dL), or hypoglycemia (glycemia < 45 mg/dL); (6) Metabolic acidosis with base excess (BE) < −10 mEq/L or serum lactate > 2 mMol/L. Neonates with two or more clinical signs and two or more laboratory signs were diagnosed as suspected EOS (Group 1) and admitted for the treatment. Blood culture positive for these newborns was considered as proven EOS (Group 2). The control group (Group 3) consisted of healthy newborns with 37–42 gestational weeks, AGA and suspicious EOS negatively detected. Maternal risk factors, demographic and perinatal data, and laboratory signs of the newborns were recorded in newborn files.
Statistical Analysis
Statistical analyses were performed using the statistical package SPSS for Windows version 22.0 (SPSS Inc., Chicago, IL, USA). The paired sample t-test and independent-sample t-test were used for continuous variables. Continuous variables were presented as the mean ± SD, and categorical variables were given as frequencies and percentages. A p-value of less than 0.05 was considered statistically significant. The performance of laboratory features in the diagnosis of EOS was calculated by using the ROC curve.
Results
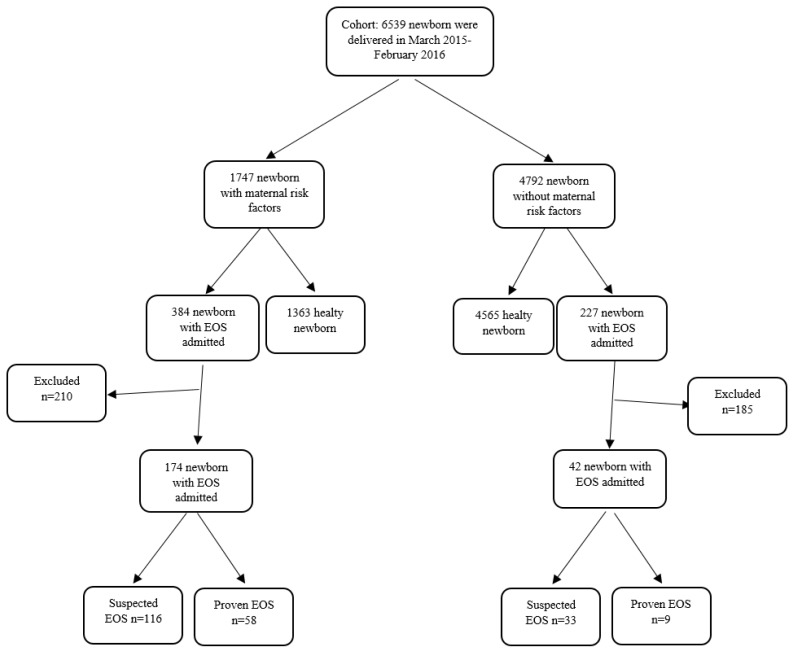
During the study period, 6,539 newborns were born in our hospital. Part of those neonates (n = 1,747) had a maternal history of urinary tract infection, vaginitis, early membrane rupture, and clinical or histological chorioamnionitis in last trimester. In addition, 384 of 1,747 neonates with maternal risk factors and 227 of 4,792 neonates without maternal risk factors were admitted to our unit with a diagnosis of EOS. Of those admitted patients, 210 of 384 newborns with maternal risk factors and 185 of 227 newborns without maternal risk factors were excluded from the study. Thus, 149 of newborns admitted with suspected EOS (Group 1), 67 proven EOS (Group 2), and 92 healthy newborns as a control group (Group 3) were included the study (Figure 1).
Figure 1.
Flowchart of the study group.
Demographic characteristics of groups are summarized in Table 1. There was no difference between groups regarding demographical and perinatal data. Comparison of hematological parameters of groups is summarized in Table 2. PLR, CRP, PCT, I/T ratio, and WBC counts were higher in group 1 and 2 compared to group 3. The mean platelet count of group 1, group 2, and group 3 were 245.7 ±66.1, 227.1 ±54.3 and 283.4 ±77.6 (Group 1–3: p = 0.98, Grup 2–3: p = 0.11), respectively. The mean lymphocyte count of group 1, group 2, and group 3 were 7.4 ± 2.1, 6.5 ±1.3 and 13.1 ±2.9 (Group 1–3: p < 0.001, Group 2–3: p < 0.001), respectively. The mean PLR of group 1, group 2, and group 3 were 56.5 ±17.8, 62.4 ±14.9 and 15.3 ±2.1 (Group 1–3: p < 0.001, Group 2–3: p < 0.001), respectively. The mean CRP values of group 1, group 2, and group 3 were 27.5 ± 6.3 mg/L, 56.9 ± 21.7 mg/L, and 4.6 ± 1.1 mg/L (Group 1–3: p < 0.001, Group 2–3: p < 0.001), respectively. The mean PCT values of group 1, group 2, and group 3 were 2.2 ± 0.09 ng/mL, 3.4 ±1.2 ng/mL, and 0.03 ± 0.01 ng/mL (Group 1–3: p < 0.001, Group 2–3: p < 0.001), respectively, and the mean I/T ratios of group 1, group 2, and group 3 were 0.25 ± 0.1, 0.33 ± 0.08, and 0.1 ± 0.05 (Group 1–3: p < 0.001, Group 2–3: p < 0.001), respectively. In suspected EOS (Group 1), PLR had an AUC of 0.812 for prediction of EOS. At a cut-off level of 39.5, RPR had a sensitivity of 88.9%, a specificity of 94.7%, a positive predictive value (PPV) of 94.3%, and a negative predictive value (NPV) of 88.6%. In proven EOS (Group 2), PLR had an AUC of 0.847 for prediction of EOS. At a cut-off level of 57.7, PLR had a sensitivity of 91.3%, a specificity of 97.6%, a PPV of 97.4%, and an NPV of 91.8%. The performance of CRP, PCT, and I/T ratio in EOS diagnosis are summarized in Table 3. The sensitivity of blood culture test was 24.8%, and the most frequently isolated microorganisms were E.coli (34.2%), coagulase negative Staphylococcus (28.9%), Staphylococcus aureus (23.6%), and Klebsiella spp (13.1%). Several CSF cultures (n = 47) obtained from 216 newborns with EOS showed no isolation.
Table 1.
Demographic characteristics of groups.
| Characteristics | Suspected EOS (n=149)Group 1 | Proven EOS (n=67)Group 2 | Control Group (n=92)Group 3 | P value |
|---|---|---|---|---|
|
| ||||
| GA, week (mean ± SD) | 38.5±4.3 | 39.1 ±5.4 | 40.1 ±3.5 | Group 1–3: >0.05 Group 2–3: >0.05 |
|
| ||||
| BW, g (mean ± SD) | 3256 ±469 | 3177 ±571 | 3487±452 | Group 1–3: >0.05 Group 2–3: >0.05 |
|
| ||||
| Gender, n (%)FemaleMale | 86 (57.7)63 (42.2) | 34 (50.7)33 (49.2) | 49 (53.2)43 (46.7) | Group 1–3: >0.05 Group 2–3: >0.05 |
|
| ||||
| Mode of Delivery, n (%)VDCS | 107 (71.8) | 49 (73.1) | 75 (81.5) | Group 1–3: >0.05 |
| 42 (28.1) | 18 (26.8) | 17 (18.4) | Group 2–3: >0.05 | |
EOS, early-onset sepsis; GA: gestational age; SD: standard deviation; BW: birth weight; g: gram; VD: vaginal delivery; CS: Caesarean section.
Table 2.
Comparison of the hematological parameters of the groups.
| Parameters | Suspected EOS (n=149)Group 1 | Proven EOS (n=67)Group 2 | Control Group (n=92)Group 3 | P value |
|---|---|---|---|---|
| Platelet count, 103/L(mean ± SD) | 245.7 ±66.1 | 237.1 ±54.3 | 283.4 ±77.6 | Group 1–3: >0.05 Group 2–3: >0.05 |
| Lymphocyte 103/L(mean ± SD) | 7.4 ±2.1 | 6.5 ±1.3 | 13.1 ±2.9 |
Group 1–3; <0.001 Group 2–3; <0.001 |
| PLR(mean ± SD) | 0.073 ±0.035 | 0.089 ±0.044 | 0.055 ±0.012 |
Group 1–3; <0.001 Group 2–3; <0.001 |
| CRP mg/L(mean ± SD) | 27.5 ±6.3 | 56.9 ±21.7 | 4.6 ±1.1 |
Group 1–3; <0.001 Group 2–3; <0.001 |
| PCT ng/mL(mean ± SD) | 2.2 ±0.09 | 3.4 ±1.2 | 0.03 ±0.01 |
Group 1–3; <0.001 Group 2–3; <0.001 |
| I/T ratio(mean ± SD) | 0.25 ±0.1 | 0.33 ±0.08 | 0.1 ±0.05 |
Group 1–3; <0.001 Group 2–3; <0.001 |
| WBC 109/L(mean ± SD) | 27.8 ±6.1 | 33.4 ±7.5 | 23.1 ±5.2 |
Group 1–3; <0.001 Group 2–3; <0.001 |
EOS, early-onset sepsis; SD: standard deviation; PLR, platelet to lymphocyte ratio; CRP, C-reactive protein; PCT, Procalcitonin; I/T, immature to total neutrophil; WBC, white blood cell.
Table 3.
The performance of different laboratory markers in EOS diagnosis.
| Parameters | AUC | Cutt of value | Sensitivity % | Specificity % | LR+ | PPV % | NPV % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | |
| PLR | 0.812 | 0.847 | 39.5 | 57.7 | 88.9 | 91.3 | 94.7 | 97.6 | 16 | 9.2 | 94.3 | 97.4 | 88.6 | 91.8 |
| CRP (mg/L) | 0.88 | 0.913 | 4.5 | 7.2 | 72.7 | 87.9 | 69.1 | 71.3 | 4.7 | 8.3 | 70.1 | 75.3 | 71.6 | 86.5 |
| PCT (ng/ml) | 0.827 | 0.865 | 0.8 | 2.2 | 69.4 | 83.4 | 96.3 | 98.8 | 11.2 | 19.3 | 94.9 | 98.5 | 75.8 | 85.6 |
| I/T ratio | 0.947 | 0.955 | 0.18 | 0.22 | 45.6 | 54.3 | 96.7 | 98.1 | 16.3 | 12.5 | 93.2 | 96.6 | 30.1 | 68.2 |
Group 1: Suspected Early Onset Neonatal Sepsis; Group 2: Proven Early Onset Neonatal Sepsis; PLR, platelet to lymphocyte ratio; CRP, C-reactive protein; PCT, Procalcitonin; I/T, immature to total neutrophil; LR+, Likelihood Ratio; PPV, Positive predictive value; NPV, Negative predictive value.
Discussion
In neonatal sepsis, early diagnosis and therapy are crucial to prevent morbidity and mortality. However, there is no excellent biomarker to use in predicting the diagnosis of NS. Many studies have been evaluating the sensitivity and specificity of the NS diagnostic markers (e.g., CRP, PCT, immature to total neutrophil ratio, CBC parameters) and results vary extensively among studies.
Celik et al., while studying the relationship between CRP and NS, evaluated the accuracy and cut-off levels of CRP and interleukin-6 (IL)-6 in the diagnosis of NS and they reported the cut-off values of CRP and IL-6 to be 4.8 mg/L and 24.65 pg/ml respectively. They determined the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for CRP to be 67%, 97%, 99%, and 39%, respectively, and for IL-6 they were 72%, 84%, 95%, and 42%, respectively.21 Cetinkaya et al. evaluated the serum amyloid A protein concentrations together with those of the CRP and PCT in the process of diagnosis and follow-up of NS in premature infants. They reported the sensitivities for CRP, PCT, and serum amyloid A to be of 72.3%, 74.8%, and 76.4%, respectively.22 In another study, Abdollahi et al. determined that the simultaneous measurement of PCT, IL-6, and high-sensitive-CRP (hs-CRP) which is more sensitive in the diagnosis of NS. They found that the combination of PCT and IL-6 had a sensitivity of 88%; PCT and hs-CRP had a sensitivity of 82%.23 In Ng et al.’s studies, the range of CRP sensitivity and specificity has been reported to be 35%–94% and 60%–96%, respectively.24 Hofer et al. investigated the relationship between CRP and early-onset neonatal sepsis (EONS). They reported that CRP values might be low due to the delay in CRP synthesis early in the development of the infection. CRP was reported to have low sensitivity during the initial hours of sepsis in previously published studies. Moreover, non-infectious factors may influence CRP kinetics; for example, delivery complications have been associated with non-specific elevations of CRP in the early perinatal period.25 Aydemir et al. studied CRP levels in clinical and proven sepsis. They reported the CRP cut-off to be 7.0 mg/L for proven sepsis. At this cut-off, the sensitivity, specificity, PPV, and NPV were 76.5%, 98.2%, 94.9%, and 90.5%, respectively. For the diagnosis of clinical sepsis, with CRP cut-off of 2.6 mg/L, the sensitivity, specificity, PPV, and NPV were 73.6%, 83.0%, 67.2%, and 86.9% respectively.26 In our study, we found the cut-off values of CRP in suspected EOS and proven EOS 4.5, 7.2 mg/L, respectively. At this cut-off, the sensitivity, specificity, PPV, and NPV in suspected and proven EOS were 72.7%, 87.9%, 69.1%, 71.3%, 70.1%, 75.3%, and 71.6%, 86.5% respectively.
PCT is physiologically produced by thyroid C-cells as a precursor of calcitonin, an acute-phase protein secreted by several tissues in response to various endogenous and exogenous stimuli such as cytokines and lipopolysaccharide, acting as a chemo-attractant factor on blood monocytes.27 In healthy neonates, plasma PCT values increase gradually after birth, reach peak values after 24 h of age (mean 1.5–2.5 ng/ml, range 0.1–20 ng/ml) and then decrease to normal values below 0.5 ng/ml by 48–72 h of age. A number of studies in children and neonates after 72 h of age, demonstrated that PCT values less than 0.5 ng/ml seem to be normal; increases to 0.5–2 ng/ml seem to be related to non-infectious inflammation, viral or focal bacterial infections; increases above a PCT value of 2–2.5 ng/ml, seem to be related to bacterial or fungal systemic infections.28–30
Some studies on the relationship between PCT and NS report that falsely high PCT levels have been detected in neonates due to non-infectious critical diseases. Moreover, normal PCT levels have been reported in severely infected newborns.31–33 Although several studies demonstrated the correlation between a low PCT level (< 2ng/ml) and Candida infection and high NPV of PCT for Candida isolation, its role in the management of antifungal treatment is far from established mainly because of the limitations in study design of supporting literature. A recently published research agenda on invasive fungal infections reported the “Utilization of PCT to guide treatment initiation and duration” as one of the ten priority for future trials in the field.34 In another study, Altunhan et al. compared the PCT levels for the diagnosis of EOS, in neonates with infectious and non-infectious processes. They did not identify the difference between the groups’ PCT levels at birth. However, the PCT levels were significantly higher in newborns with suspected sepsis at 24 h of age, and at a cut-off value of 5.3 ng/mL. They determined that the specificity, sensitivity, PPV, and NPV were all increased compared to the cut-off value of 0.59 ng/mL at birth.35 In our study, we found the cut-off values of PCT in suspected EOS and proven EOS 0.8, 2.2 mg/L, respectively. At this cut-off, the sensitivity, specificity, PPV, and NPV in suspected and proven EOS were 69.4%, 83.4%, 96.3%, 98.8%, 94.9%, 98.5%, and 75.8%, 85.6% respectively.
A number of the studies have explored the role of various parameters of complete blood count on the diagnosis of neonatal sepsis, e.g., white blood cell count (WBC), absolute neutrophil count (ANC), immature/total leucocyte ratio (I:T ratio), MPV, RDW, PDW, neutrophil and lymphocyte count. Hornik et al. reported that low WBC count, low ANC, and high I: T ratio were associated with a higher risk of infection and that these markers have high specificity and NPV but low sensitivity.36 Murphy et al. reported that the combination of two consecutive normal I: T ratio results and a sterile blood culture has 100% NPV.37 Shaaban et al. were investigating MPV value as a diagnostic tool in early-onset neonatal sepsis (EOS). They reported that MPV was found to be higher in the sepsis group and sensitivity and specificity on MPV were 97.1% and 100%, respectively.38 Patrick et al. evaluated 156 newborns and demonstrated that MPV measurements were considerably higher in patients with bacteremia than in newborns without infection. The authors reported the MPV sensitivity and specificity for the diagnosis of sepsis to be 42% and 95%, respectively.39 Zhang et al. studied the utility of red cell distribution width (RDW), platelet distribution width (PDW), neutrophil-lymphocyte count ratio (NLCR), PCT, and CRP in the diagnosis of neonatal sepsis NS. They found that PCT has the highest sensitivity (91.7%), and PDW has the highest specificity (84.7%).40 In this study, RDW, PDW, NLCR have a sensitivity of 73.3%, 38.3%, and 81.1%; a specificity of 49.2%, 84.7%, and 62.7%; a PPV of 59.1%, 71.5%, and 68.5%, and a NPV of 64.8%, 57.9%, and 76.8%, respectively.
The physiological immune response of circulating leukocytes to numerous stressful events is characterized by a raised neutrophil count and decreased lymphocyte count. A microbial infection causes an increase of the total leukocyte and neutrophil counts and results in an inflammatory reaction. For this reason, these counts might be used as diagnostic markers of microbial infection.41–42 Platelet to lymphocyte ratio (PLR) is a new and easily calculated value, and it is proven to have a high predictive value at diagnosis of inflammatory diseases in adults.15–18 Our study’s goal was identifying the utility of PLR in the prediction and suspicious diagnosis of early-onset neonatal sepsis. There is only one study on PLR in neonatal sepsis so far, to the best of our knowledge. Can et al. reported that a neutrophil to lymphocyte ratio (NLR) of 6.76 was the predictive cut-off value of EOS (sensitivity 97.4%; specificity 100%; AUROC curve 0.99; P=0.001), and a PLR of 94.05 was determined as the predictive cut-off value of EOS (sensitivity 97.4%; specificity 100%; AUROC curve 0.93; P=0.001).43 In our study, we identified that the PLR levels of suspected and definite EOS were significantly higher than that of the control group. PLR value of neonates with suspected EOS had a cut-off level of 39.5, 88.9% sensitivity, 94.7% specificity, 94.3% PPV, and 88.6% NPV. PLR value in neonates with definite EOS had a cut-off level of 57.7, 91.3% sensitivity, 97.6% specificity, 97.4% PPV, and 91.8% NPV. CRP, in suspected and definite EOS, had a cut-off level of 4.5–7.2 mg/L, 72.7%–87.9% sensitivity, 79.1%–81.3% specificity, 94.9%–98.5% PPV, and 75.8%–85.6% NPV, respectively. PCT in suspected and definite EOS had a cut-off level of 0.8–2.2 ng/mL, 69.4%–83.4% sensitivity, 96.3%–98.8% specificity, 77.6%–82.4% PPV, and 76.9%–87% NPV, respectively. It was confirmed that PLR has a higher specificity and PPV in comparison with other biomarkers used in the diagnosis of EOS.
Furthermore, sensitivity, specificity, PPV, and NPV values of PLR were found to be higher than CRP and PCT. Based on these findings of our study, we conclude that PLR is cost-effective, easily calculated, needs a small amount of blood, is an easy test to perform, and has high sensitivity, specificity, PPV, and NPV values. We determined that PLR is a reliable marker to be used in the early prediction of EONS and maybe a good alternative to others, currently used parameters.
The strengths of our study include a large sample size and the point that it compared suspected and definite EOS, proven EOS, and assigning a control group. Our study also has some limitations: first, it was performed retrospectively; second, even though we excluded patients with other inflammatory diseases, accompanying inflammatory comorbidities may have influenced the reliability of the results. Of course, the specificity of this test has been evaluated in the context of strict adherence to the criteria adopted in choosing the subjects studied.
To summarize, identifying a biomarker with a high predictive value is significance for early diagnosis, treatment, and prevention of NS. Based on our results, we consider that PLR can be used as a new biomarker in the early detection of EOS.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015;100(3):F257–F263. doi: 10.1136/archdischild-2014-306213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polin RA F Committee on, Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(5):1006–1015. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J, Vincent JL, Adhikari NKJ, Machado FR, Angus DC, Calandra T, et al. Sepsis: A roadmap for future research. The Lancet Infectious Diseases. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 4.Gerdes JS. Diagnosis and management of bacterial infections in the neonate. Pediatr Clin N Am. 2004;51(4):939–959. doi: 10.1016/j.pcl.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay S, Taylor JA, Von Kohorn I, et al. Variation in sepsis evaluation across a national network of nurseries. Pediatrics. 2017;139(3) doi: 10.1542/peds.2016-2845. [DOI] [PubMed] [Google Scholar]
- 6.Mussap M, Noto A, Cibecchini F, Fanos V. The importance of biomarkers in neonatology. Semin Fetal Neonatal Med. 2013;18:56–64. doi: 10.1016/j.siny.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Jyothi P, Basavaraj MC, Basavaraj PV. Bacteriological profile of neonatal septicemia and antibiotic susceptibility pattern of the isolates. J Nat Sci Biol Med. 2013;4:306–9. doi: 10.4103/0976-9668.116981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benitz WE. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol. 2010;37:421–38. doi: 10.1016/j.clp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451(Pt A):46–64. doi: 10.1016/j.cca.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Su H, Chang SS, Han CM, et al. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis-a systemic review and meta-analysis. J Perinatol. 2014;34:268–74. doi: 10.1038/jp.2013.186. [DOI] [PubMed] [Google Scholar]
- 11.Zhou M, Cheng S, Yu J, et al. Interleukin-8 for diagnosis of neonatal sepsis: a meta-analysis. PLoS One. 2015;10:e0127170. doi: 10.1371/journal.pone.0127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laborada G, Rego M, Jain A, Guliano M, Stavola J, Ballabh P, et al. Diagnostic value of cytokines and C-reactive protein in the first 24 hours of neonatal sepsis. Am J Perinatol. 2003;20:491–501. doi: 10.1055/s-2003-45382. doi: 10.1055/s-2003-45382. 5. [DOI] [PubMed] [Google Scholar]
- 13.Chiesa C, Pacifico L, Osborn JF, Bonci E, Hofer N, Resch B. Early-onset neonatal sepsis: still room for improvement in procalcitonin diagnostic accuracy studies. Medicine (Baltimore) 2015;94:e1230. doi: 10.1097/MD.0000000000001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedegaard SS, Wisborg K, Hvas A-M. Diagnostic utility of biomarkers for neonatal sepsis-a systematic review. Infect Dis (Lond) 2015;47:117–124. doi: 10.3109/00365548.2014.971053. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Peng S, Wang A, Xie H, Guo L, Jiang N, Niu Y. Platelet-lymphocyte ratio acts as an independent predictor of prognosis in patients with renal cell carcinoma. Clin Chim Acta. 2018 May;480:166–172. doi: 10.1016/j.cca.2018.02.014. Epub 2018 Feb 17. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Xue H, Liu W, Wu F, Wang Y, Gao H. Meta-analysis of Platelet Lymphocyte Ratio as A Prognostic Factor for Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi. 2019 May 20;22(5):289–298. doi: 10.3779/j.issn.1009-3419.2019.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y, Huang X, Zhang W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: interaction effect with disease severity-a retrospective study. BMJ Open. 2019 Jan 25;9(1):e022896. doi: 10.1136/bmjopen-2018-022896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, et al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediators Inflamm. 2018 Jul 15;2018 doi: 10.1155/2018/3758068. 3758068. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency (EMA) Report on the Expert Meeting on Neonatal and Pediatric Sepsis. 2010. [Google Scholar]
- 21.Celik IH, Demirel FG, Uras N, et al. What are the cut-off levels for IL-6 and CRP in neonatal sepsis? J Clin Lab Anal. 2010;24:407–12. doi: 10.1002/jcla.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cetinkaya M, Ozkan H, Köksal N, et al. Comparison of serum amyloid A concentrations with those of C-reactive protein and procalcitonin in diagnosis and follow-up of neonatal sepsis in premature infants. J Perinatol. 2009;29:225–31. doi: 10.1038/jp.2008.207. [DOI] [PubMed] [Google Scholar]
- 23.Abdollahi A, Shoar S, Nayyeri F, Shariat M. Diagnostic Value of Simultaneous Measurement of Procalcitonin, Interleukin-6 and hs-CRP in Prediction of Early-Onset Neonatal Sepsis. Mediterr J Hematol Infect Dis. 2012;4:e2012028. doi: 10.4084/mjhid.2012.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opin Pediatr. 2006;18:125–31. doi: 10.1097/01.mop.0000193293.87022.4c. [DOI] [PubMed] [Google Scholar]
- 25.Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102:25–36. doi: 10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- 26.Aydemir C, Aydemir H, Kokturk F, Kulah C, Mungan AG. The cut-off levels of procalcitonin and C-reactive protein and the kinetics of mean platelet volume in preterm neonates with sepsis. BMC Pediatr. 2018;18:253. doi: 10.1186/s12887-018-1236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fendler WM, Piotrowski AJ. Procalcitonin in the early diagnosis of nosocomial sepsis in preterm neonates. J Paediatr Child Health. 2008;44(3):114–8. doi: 10.1111/j.1440-1754.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 28.Chiesa C, Panero A, Rossi N, Stegagno M, De Giusti M, Osborn JF, Pacifico L. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–72. doi: 10.1086/514576. [DOI] [PubMed] [Google Scholar]
- 29.Gendrel D, Assicot M, Raymond J, Moulin F, Francoual C, Badoual J, Bohuon C. Procalcitonin as a marker for the early diagnosis of neonatal infection. J Pediatr. 1996;128(4):570–3. doi: 10.1016/S0022-3476(96)70374-8. [DOI] [PubMed] [Google Scholar]
- 30.van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis. 2004;4(10):620–30. doi: 10.1016/S1473-3099(04)01146-6. [DOI] [PubMed] [Google Scholar]
- 31.Monneret G, Labaune JM, Isaac C, Bienvenu F, Putet G, Bienvenu J. Procalcitonin and C-reactive protein levels in neonatal infections. Acta Paediatr. 1997;86:209–12. doi: 10.1111/j.1651-2227.1997.tb08870.x. [DOI] [PubMed] [Google Scholar]
- 32.Lapillonne A, Basson E, Monneret G, Bienvenu J, Salle BL. Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet. 1998;351:1211–2. doi: 10.1016/S0140-6736(05)79165-0. [DOI] [PubMed] [Google Scholar]
- 33.Chiesa C, Panero A, Rossi N, Stegagno M. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26:664–72. doi: 10.1086/514576. [DOI] [PubMed] [Google Scholar]
- 34.Bassetti M, Garnacho-Montero J, Calandra T, Kullberg B, Dimopoulos G, Azoulay E, Chakrabarti A, Kett D, Leon C, Ostrosky-Zeichner L, Sanguinetti M, Timsit JF, Richardson MD, Shorr A, Cornely OA. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med. 2017 doi: 10.1007/s00134-017-4731-2. [DOI] [PubMed] [Google Scholar]
- 35.Altunhan H, Annagür A, Örs R, Mehmetoğlu I. Procalcitonin measurement at 24 hours of age may be helpful in the prompt diagnosis of early-onset neonatal sepsis. Int J Infect Dis. 2011;15:e854–8. doi: 10.1016/j.ijid.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Hornik CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31:799–802. doi: 10.1097/INF.0b013e318256905c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy K, Weiner J. Use of leukocyte counts in evaluation of early-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31:16–19. doi: 10.1097/INF.0b013e31822ffc17. [DOI] [PubMed] [Google Scholar]
- 38.Shaaban HA, Safwat N. Mean Platelet Volume in Preterm: A Predictor of Early Onset Neonatal Sepsis. J Matern Fetal Neonatal Med. 2018;22:1–6. doi: 10.1080/14767058.2018.1488161. [DOI] [PubMed] [Google Scholar]
- 39.Patrick CH, Lazarchick J. The effect of bacteremia on automated platelet measurement in neonates. Am J Clin Pathol. 1990;93:391–4. doi: 10.1093/ajcp/93.3.391. [DOI] [PubMed] [Google Scholar]
- 40.Zang HB, Chen J, Lan FQ, Ma XJ, Zhang SY. Diagnostic values of red cell distribution width, platelet distribution width and neutrophil-lymphocyte count ratio for sepsis. Experimental and Therapeutic Medicine. 2016;12:2215–2219. doi: 10.3892/etm.2016.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elawady S, Botros SK, Sorour AE, et al. Neutrophil CD64 as a Diagnostic Marker of Sepsis in Neonates. J Investig Med. 2014;62:644–9. doi: 10.2310/JIM.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 42.Yoon NB, Son C, Um SJ. Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia. Ann Lab Med. 2013;33:105–110. doi: 10.3343/alm.2013.33.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Can E, Hamilcikan Ş, Can C. The Value of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio for Detecting Early-onset Neonatal Sepsis. J Pediatr Hematol Oncol. 2018 May;40(4):e229–e232. doi: 10.1097/MPH.0000000000001059. [DOI] [PubMed] [Google Scholar]