Abstract
Background
Recent artemisinin-combination therapy failures in Cambodia prompted a search for alternatives. Atovaquone‐proguanil (AP), a safe, effective treatment for multidrug-resistant Plasmodium falciparum (P.f.), previously demonstrated additive effects in combination with artesunate (AS).
Methods
Patients with P.f. or mixed-species infection (n = 205) in Anlong Veng (AV; n = 157) and Kratie (KT; n = 48), Cambodia, were randomized open-label 1:1 to a fixed‐dose 3-day AP regimen +/-3 days of co‐administered artesunate (ASAP). Single low-dose primaquine (PQ, 15 mg) was given on day 1 to prevent gametocyte-mediated transmission.
Results
Polymerase chain reaction–adjusted adequate clinical and parasitological response at 42 days was 90% for AP (95% confidence interval [CI], 82%–95%) and 92% for ASAP (95% CI, 83%–96%; P = .73). The median parasite clearance time was 72 hours for ASAP in AV vs 56 hours in KT (P < .001) and was no different than AP alone. At 1 week postprimaquine, 7% of the ASAP group carried microscopic gametocytes vs 29% for AP alone (P = .0001). Nearly all P.f. isolates had C580Y K13 propeller artemisinin resistance mutations (AV 99%; KT 88%). Only 1 of 14 treatment failures carried the cytochrome bc1 (Pfcytb) atovaquone resistance mutation, which was not present at baseline. P.f. isolates remained atovaquone sensitive in vitro but cycloguanil resistant, with a triple P.f. dihydrofolate reductase mutation.
Conclusions
Atovaquone-proguanil remained marginally effective in Cambodia (≥90%) with minimal Pfcytb mutations observed. Treatment failures in the presence of ex vivo atovaquone sensitivity and adequate plasma levels may be attributable to cycloguanil and/or artemisinin resistance. Artesunate co-administration provided little additional blood-stage efficacy but reduced post-treatment gametocyte carriage in combination with AP beyond single low-dose primaquine.
Keywords: atovaquone-proguanil, artesunate, drug resistance, malaria, primaquine
Despite no additional asexual-stage malaria efficacy, adding artesunate (AS) to 3-day atovaquone-proguanil (AP) treatment reduced post-treatment gametocyte carriage in Cambodia. AP monotherapy retained marginally > 90% efficacy against multidrug resistant P. falciparum. Only one recurrent isolate harbored Pfcytb atovaquone resistance mutation.
Treatment failures of multiple Plasmodium falciparum drugs have accelerated over the past decade in Cambodia. Most recently, substantial rates of clinical dihydroartemisinin-piperaquine combination therapy failures emerged within just 3 years of introduction [1], with confirmed resistance to both components [2]. In response, health authorities issued guidelines reinstating artesunate-mefloquine combinations as firstline agents in affected areas [3]. These were based on ex vivo surveillance data indicating inverse resistance patterns between piperaquine and mefloquine [4, 5] attributed to Pfmdr1 mutations [6]. This was intended as a stop-gap public health measure pending more effective therapies.
Unfortunately, few viable alternatives currently exist, prompting calls for more intensive nonpharmacologic approaches including universal directly observed inpatient treatment [7] and military mobilization [8]. Atovaquone-proguanil (AP), a fixed-dose combination that synergistically uncouples parasite mitochondria, remains one of the few remaining alternative therapies, with limited use to date as part of a World Health Organization (WHO) containment program in Pailin province [9] and more recently as a second-line therapy in Thailand. Preserved ex vivo efficacy was confirmed in areas of recent dihydroartemisinin-piperaquine failure [10]. AP was primarily developed as a well-tolerated daily “causal” liver-stage prophylaxis drug, but is also effective against multidrug-resistant (MDR) malaria [11]. The primary disadvantage of treating P.f. malaria with AP is rapid in-host selection of cytochrome-bc1 parasite mutations conferring clinical resistance, though resistance without bc1 mutations has also been described [12].
A prior study in Thailand indicated that the combination of artesunate with atovaquone-proguanil (ASAP) (Atlantic Laboratories Corp., Ltd., Bangkok, Thailand) is more effective than AP alone while also reducing subsequent gametocyte carriage [13]. In addition to added efficacy, it is possible that the combination may delay or prevent further development of resistance to the individual agents, given varying mechanisms of action. We tested the efficacy of AP with and without AS and evaluated the effects of single low-dose primaquine (15 mg) (The Government Pharmaceutical Organization (GPO), Bangkok, Thailand) paired with the 2 combinations on gametocyte carriage post-treatment.
METHODS
Study Design and Participants
Cambodian volunteers with uncomplicated P.f. or mixed P. falciparum/P. vivax malaria aged 18–65 years were enrolled in a 2-arm open label treatment trial at Anlong Veng Referral Hospital, Oddar Meanchey Province, and Kratie Provincial Referral Hospital. Patients with 100–200 000 parasites/μL of blood were included, whereas patients with severe malaria, allergy, contraindication to study drugs, use of antimalarials in the previous 7 days (or atovaquone-proguanil in the previous 30), and pregnant or lactating women were excluded. Participants provided written informed consent in Khmer. The study protocol was approved by US and Cambodian regulatory authorities as study WR2115 (ClinicalTrials.gov NCT02297477).
Procedures
Patients were treated in-hospital under directly observed therapy. Volunteers were randomly assigned to 3 days of atovaquone-proguanil (AP; 1000 mg/400 mg) with or without 200 mg of artesunate (ASAP) daily with 1:1 allocation using time-blocked randomization with a block size of 4. Volunteers selected treatment assignment codes from a collection of sealed envelopes to mask allocation. Based on published international and local guidance by the WHO at the time, all volunteers received 15 mg of primaquine on day 1, regardless of G6PD status. Microscopists were blinded to each other’s readings and to study drug regimen.
All investigational products met quality standards for weight and content uniformity by UPLC-MS analysis before the study [14]. Volunteer glucose-6-phosphate-dehydrogenase (G6PD) activity was evaluated by a fluorescence spot test (R&D Diagnostics Ltd., Greece).
Vital signs were taken at 0, 4, and 8 hours after the first dose, then every 8 hours until discharge. Giemsa-stained thick and thin malaria smears [15] were performed at 4 and 8 hours after first study drug dose, then every 8 hours until 2 consecutive negative smears. Gametocytemia was evaluated per 2000 white blood cell count (WBC) on thin smear, whereas asexual-stage parasitemia was per 500 WBC [16]. Real-time polymerase chain reaction (PCR) detection of malaria was performed at 0, 24, 48, and 72 hours, then weekly and at recurrence [17]. Atovaquone levels were collected at 0, 4, 24, 48, and 72 hours, weeks 1 and 2, and recurrence. An ultraperformance liquid chromatography (UPLC) coupled with tandem mass spectrometry (MS/MS) method in human plasma with carboxymefloquine as an internal standard was developed, optimized, and validated based on US Food and Drug Administration guidance. Plasma atovaquone concentration was measured using a Waters Acquity UPLC BEH C18, 2.1×50 mm, 1.8-µm column with a gradient mobile phase of 5 mM of ammonium acetate in water (pH 7.0) and 5 mM of ammonium acetate in acetonitrile at a flow rate of 0.4 mL/min over 5 minutes. Plasma samples were extracted by acetonitrile protein precipitation with known concentrations of carboxymefloquine. Clear supernatants were transferred to UPLC vials after vortexing and centrifugation for 10 minutes. Selective mass/charge (m/z) transitions were monitored for atovaquone (365.04 to 337.02) and carboxymefloquine (307.94 to 223.93).
Volunteers were monitored throughout the study for adverse events using Common Terminology Criteria for Adverse Events [18], including performance of complete blood count and renal and liver function tests at 0, 48, and 72 hours. After discharge, outpatient clinical follow-up continued for 42 days, with weekly malaria smears. Females had urine pregnancy tests at baseline, recurrence, and weeks 2, 4, and 6. Recurrent malaria was treated following current national treatment guidelines.
In cases of malaria recurrence, recrudescence was determined by P.f. msp1, msp2, and glurp genotyping [19]. Molecular markers of resistance were assessed at baseline and recurrence, including the K13 propeller marker for artemisinins [1], the cytochrome bc1 atovaquone mutation [10], and dihydrofolate reductase (DHFR) mutations associated with cycloguanil resistance, an active metabolite of proguanil [20]. Sequencing for AS-resistant Pf Kelch 13 variants covered the entire pfk13 propeller domain, including previously described mutations at R561H, N537I, P553L, G449A, and C580Y. In these Cambodian isolates, Pf kelch mutation was only found at position C580Y. Additionally, the entire mitochondrial cytochrome b gene was sequenced by amplifying a 2.7-kb mtDNA fragment using the Sanger method with 8 different sequencing primers (Supplementary Table 1). Immediate ex vivo drug susceptibility of fresh P.f. isolates to commonly used antimalarials was measured as 50% inhibitory concentration (IC50) [1] based on histidine-rich protein 2 (HRP-2) enzyme-linked immunosorbent assay (ELISA) [10, 21].
Outcomes
The primary outcome was PCR-adjusted adequate clinical and parasitological response (ACPR), defined as absence of clinical or parasitological malaria recurrence within 42 days of follow-up.
Statistical Analysis
Prior efficacy point estimates from a large (n = 1596) comparative study conducted in Thailand a decade earlier [13] were 97% for AP and 99% for ASAP. Sample size of 100 in each treatment arm was calculated to determine 95% confidence intervals for each treatment arm independently around a 90% 42-day per-protocol efficacy for AP and 95% for ASAP. These were considered to be acceptable thresholds to support ongoing use as tertiary regimens in the national treatment program. Data were double-entered into Microsoft Access 2007, with random 20% reverification by the clinical study monitor, and analyzed using STATA, version 15.0 (Stata Corp, College Station, TX), and GraphPad Prism, version 6.07. All patients receiving at least 1 dose of study drug were included in the intention-to-treat analysis, whereas per-protocol analysis excluded all withdrawn or censored volunteers.
Cumulative risk of treatment failure was assessed with Kaplan-Meier survival over 42 days using log-rank comparison tests. Cox proportional hazards models were used to assess clinical and molecular covariates and recrudescence. Comparisons of categorical variables were made with the Fisher exact test, whereas continuous variables were compared with the Student t test or Wilcoxon rank-sum test (for non–normally distributed data). A 2-sided P value of less than 0.05 was considered significant.
RESULTS
Study Population
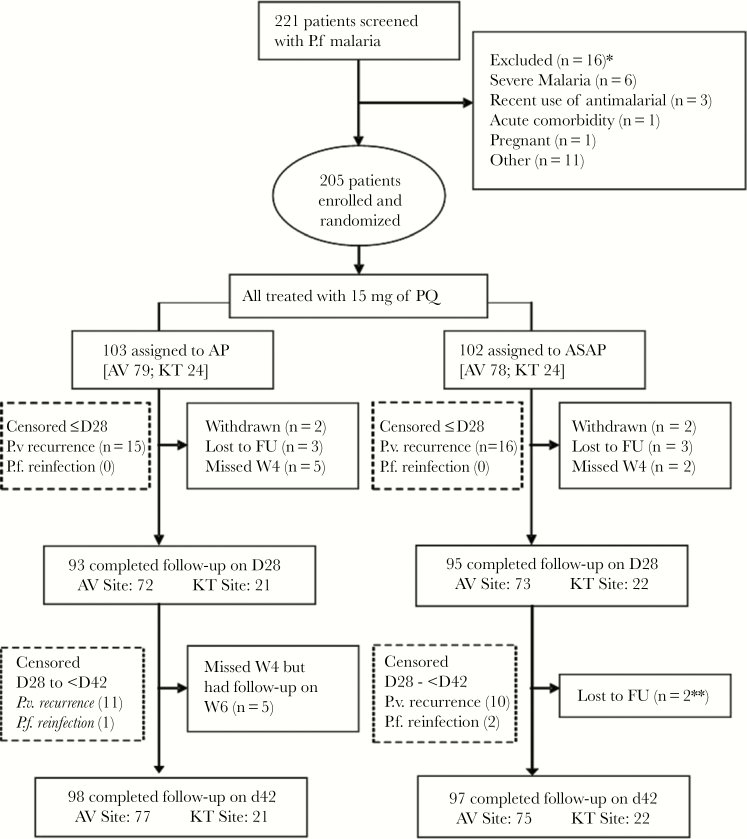
Of 221 patients screened with acute P.f. or mixed malaria infection, 205 (93%) were enrolled and randomized, with 95% completing 42-day follow-up (Figure 1). One hundred fifty-seven volunteers were enrolled in northern Cambodia (Anlong Veng) and 48 in eastern Cambodia (Kratie), mostly symptomatic adult males. Patient baseline characteristics were similar between treatment arms and study sites (Table 1). Less than 8% had preexisting plasma antimalarial activity detected at baseline (Table 2).
Figure 1.
CONSORT flow diagram. Abbreviations: AP, atovaquone-proguanil; ASAP, artesunate-atovaquone-proguanil; AV, Anlong Veng; FU, follow-up; KT, Kratie; PQ, primaquine.
Table 1.
Baseline Characteristics of Study Subjects
| Baseline Characteristics | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | ASAP | AP | Sig. | Anlong Veng | Kratie | Sig. | Total |
| Total, No. | 102 | 103 | 157 | 48 | 205 | ||
| Male sex, No. (%) | 99 (97.1) | 100 (97.1) | 0.990 | 152 (96.8) | 47 (97.9) | 0.692 | 199 (97.1) |
| Age, mean (IQR), y | 31.2 (16) | 29.4 (15) | 0.180 | 30.1 (14) | 30.8 (16) | 0.652 | 30.3 (14) |
| Age, median (IQR), y | 28.0 (16) | 28.0 (15) | 0.275 | 28 (14) | 28 (16) | 0.951 | 28 (14) |
| Khmer ethnicity, No. (%) | 102 (100) | 103 (100) | - | 157 (100) | 48 (100) | - | 205 (100) |
| Occupation, No. (%) | |||||||
| Farmer | 94 (92.2) | 97 (94.2) | 0.567 | 145 (92.4) | 46 (95.8) | 0.403 | 191 (93.2) |
| Military | 7 (6.9) | 5 (4.9) | 0.540 | 12 (7.6) | 0 (0.0) | 0.048 | 12 (5.8) |
| Other | 1 (1.0) | 1 (1.0) | 0.994 | 0 (0.0) | 2 (4.2) | 0.010 | 2 (1.0) |
| Weight, mean (SD), kg | 56.0 (7.1) | 55.1 (6.5) | 0.337 | 55.6 (6.7) | 55.4 (7.0) | 0.862 | 55.6 (6.8) |
| Body mass index, No. (%) | |||||||
| Underweight <18.5 kg/m2 | 9 (8.8) | 13 (12.6) | 0.380 | 14 (8.9) | 8 (16.7) | 0.129 | 22 (10.7) |
| Normal 18.5–24.9 kg/m2 | 88 (86.3) | 87 (84.5) | 0.714 | 138 (87.9) | 37 (77.1) | 0.064 | 175 (85.4) |
| Overweight 25–29.9 kg/m2 | 5 (4.9) | 3 (2.9) | 0.462 | 5 (3.2) | 3 (6.3) | 0.337 | 8 (3.9) |
| Symptoms, No. (%) | |||||||
| Fever | 101 (99.0) | 103 (100) | 0.314 | 156 (99.4) | 48 (100) | 0.579 | 204 (99.5) |
| Headache | 99 (97.1) | 103 (100) | 0.080 | 155 (98.7) | 47 (97.9) | 0.683 | 202 (98.5) |
| Muscle aches | 62 (60.8) | 61 (59.2) | 0.820 | 103 (65.6) | 20 (41.7) | 0.003 | 123 (60.0) |
| Chills | 85 (83.3) | 85 (82.5) | 0.878 | 124 (79.0) | 46 (95.8) | 0.007 | 170 (82.9) |
| Abdominal pain | 26 (25.5) | 30 (29.1) | 0.559 | 51 (32.5) | 5 (10.4) | 0.003 | 56 (27.3) |
| Dizziness | 18 (17.6) | 24 (23.3) | 0.316 | 27 (17.2) | 15 (31.3) | 0.035 | 42 (20.5) |
| Fatigue | 20 (19.6) | 18 (17.5) | 0.694 | 26 (16.6) | 12 (25.0) | 0.188 | 38 (18.5) |
| Nausea | 11 (10.8) | 11 (10.7) | 0.981 | 12 (7.6) | 10 (20.8) | 0.010 | 22 (10.7) |
| Vomit | 1 (1.0) | 2 (1.9) | 0.567 | 2 (1.3) | 1 (2.1) | 0.683 | 3 (1.5) |
| Diarrhea | 1 (1.0) | 1 (1.0) | 0.994 | 2 (1.3) | 0 (0.0) | 0.432 | 2 (1.0) |
| Anorexia | 1 (1.0) | 0 (0.0) | 0.314 | 0 (0.0) | 1 (2.1) | 0.070 | 1 (1.0) |
| Temperature, mean (SD), ℃ | 37.9 (0.8) | 37.7 (0.9) | 0.124 | 37.8 (0.8) | 38.0 (1.0) | 0.138 | 37.8 (0.8) |
| Duration of fever, median (IQR), d | 2 (1) | 2 (1) | 0.542 | 2 (2) | 3 (1) | 0.000 | 2 (1) |
| History of previous malaria episode, No. (%) | 64 (62.7) | 71 (68.9) | 0.350 | 114 (72.6) | 21 (43.8) | 0.000 | 135 (65.9) |
| History of malaria medication, No. (%) | |||||||
| ≥1–<2 wk | 1 (1.0) | 0 (0.0) | 0.314 | 0 (0.0) | 1 (2.1) | 0.070 | 1 (0.5) |
| ≥2–<4 wk | 2 (2.0) | 10 (9.7) | 0.018 | 12 (7.6) | 0 (0.0) | 0.048 | 12 (5.9) |
| ≥1–<3 mo | 33 (32.4) | 42 (40.8) | 0.211 | 59 (37.6) | 16 (33.3) | 0.593 | 75 (36.6) |
| ≥3–<6 mo | 12 (11.8) | 12 (11.7) | 0.980 | 23 (14.6) | 1 (2.1) | 0.018 | 24 (11.7) |
| ≥6–<12 mo | 16 (15.7) | 7 (6.8) | 0.044 | 20 (12.7) | 3 (6.3) | 0.213 | 23 (11.2) |
| Hepatomegaly, No. (%) | 0 (0.0) | 1 (1.0) | 0.318 | 1 (0.6) | 0 (0.0) | 0.579 | 1 (0.5) |
| Splenomegaly, No. (%) | 1 (1.0) | 1 (1.0) | 0.994 | 1 (0.6) | 1 (2.1) | 0.372 | 2 (1.0) |
| Parasitemia, geomean (5, 95 centiles), /µL | 7700.1 (909, 62921) | 7730.8 (832, 93188) | 0.259 | 7491.8 (841, 76661) | 8494.9 (310, 88519) | 0.168 | 7715.5 (832, 76661) |
| Parasite density group, No. (%) | |||||||
| <1000/µL | 8 (7.8) | 9 (8.7) | 0.816 | 12 (7.6) | 5 (10.4) | 0.542 | 17 (8.3) |
| ≥1000 and ≤10 000/µL | 49 (48.0) | 53 (51.5) | 0.625 | 81 (51.6) | 21 (43.8) | 0.342 | 102 (49.8) |
| >10 000 and ≤100 000/µL | 45 (44.1) | 37 (35.9) | 0.231 | 61 (38.9) | 21 (43.8) | 0.545 | 82 (40.0) |
| >100 000/µL | 0 (0.0) | 4 (3.9) | 0.044 | 3 (1.9) | 1 (2.1) | 0.940 | 4 (1.9) |
| Presence of Pf gametocytes, No. (%) | 24 (23.5) | 18 (17.5) | 0.283 | 33 (21.0) | 9 (18.8) | 0.733 | 42 (20.5) |
| Creatinine clearance, mean (SD) | 94.1 (19.8) | 94.8 (18.9) | 0.785 | 96.9 (18.7) | 86.5 (19.4) | 0.001 | 94.5 (19.3) |
| White cell count, median (IQR), ×103/µL | 6.1 (3.4) | 6.5 (2.6) | 0.368 | 6.5 (2.8) | 6.1 (3.1) | 0.084 | 6.4 (2.9) |
| Red cell count, median (IQR), ×106/µL | 4.7 (0.9) | 4.9 (0.9) | 0.043 | 4.8 (0.8) | 5.1 (0.9) | 0.004 | 4.8 (0.8) |
| Hemoglobin, mean (SD), g/dL | 12.7 (1.6) | 13.0 (1.7) | 0.203 | 12.8 (1.6) | 13.3 (1.7) | 0.031 | 12.9 (1.6) |
| Hematocrit, mean (SD) | 37.8 (4.6) | 38.7 (4.8) | 0.161 | 37.4 (4.4) | 41.0 (4.7) | 0.000 | 38.2 (4.7) |
| Platelet count, median (IQR), ×103/µL | 139 (62) | 143 (70) | 0.929 | 147 (65) | 128 (62) | 0.004 | 141 (66) |
| Absolute neutrophil count, median (IQR), ×103/µL | 4.0 (2.8) | 4.2 (2.9) | 0.556 | 4.3 (2.9) | 3.6 (2.8) | 0.154 | 4.2 (2.8) |
| G6PD deficiency, No. (%) | 12 (11.8) | 17 (16.5) | 0.330 | 25 (15.9) | 4 (8.3) | 0.187 | 29 (14.1) |
Abbreviations: AP, atovaquone-proguanil; ASAP, artesunate-atovaquone-proguanil; IQR, interquartile range. Bolded text refers to statistically significant results.
Table 2.
Efficacy Outcomes for Atovaquone-Proguanil vs Artesunate-Atovaquone-Proguanil in Cambodia
| Treatment Arm | Northern Cambodia (Anlong Veng, Oddar Meanchey Province) | Eastern Cambodia (Kratie Province) | |||||
|---|---|---|---|---|---|---|---|
| AP (n = 103) | ASAP (n = 102) | P | AP (n = 79) | ASAP (n = 78) | AP (n = 24) | ASAP (n = 24) | |
| Preexisting antimalarial activity before treatment e | 8 (7.77) [3.41–14.7] | 4 (3.9) [1.08–9.74] | .241 | 7 (8.9) [3.64–1.74] | 4 (5.1) [1.41–12.6] | 1 (4.2) [0.10–21.1] | 0 (0) [0–14.3] |
| Mixed P.f./P.v. infection at enrollment | 6 (5.8) [2.16–12.2] | 8 (7.8) [3.44–14.9] | .567 | 4 (5.1) [1.40–12.5] | 6 (7.7) [2.87–16.0] | 2 (8.3) [1.03–27.0] | 2 (8.3) [1.03–27.0] |
| K13 propeller C580Y mutation | 99/101 (98) [93.0–99.8] | 95/99 (96) [90.0–99.0] | .443 | 79 (100) | 77 (99) | 20/22 (91) | 18/21 (86) |
| Parasitemia positive on D3 | |||||||
| Positive by microscopy | 37 (36) [26.7–46.0] | 34 (33) [24.3–43.4] | .700 | 33 (42) | 33 (42) | 4 (17) | 1 (4) |
| Positive by quantitative PCR | 74 (71.8) [62.1–80.3] | 77 (75.5) [66.0–83.5] | .554 | 54 (68) | 60 (77) | 20 (83) | 17 (74) |
| Parasite clearance time, median (IQR), h | 72 (32–104) | 64 (16–104) | .250 | 72 (32–104) | 72 (16–104) | 68 (32–88) | 56 (32–96) |
| Median time to PCT 50 | 17 (0.8–47) | 9.5 (0.4–38) | <.0001 | 18 (0.8–47) | 9.6 (0.4–38) | 15 (3.6–36) | 9.4 (1.2–23) |
| Median time to PCT 90 | 31 (12–63) | 22 (3.2–55) | <.0001 | 33 (12–63) | 23 (3.2–55) | 29 (16–44) | 18 (12–35) |
| Median time to PCT 95 | 37 (16–71) | 29 (4.4–62) | <.0001 | 39 (16–71) | 29 (4.4–62) | 35 (20–47) | 23 (6–41) |
| Recrudescence PCT <72 h & PCT 1/2 <5 h | 2/8 (25) | 0/6 (0) | .473 | 2/13 (15) | 0/1 (0) | ||
| Recrudescence PCT ≥72 h & PCT 1/2 ≥5 h f | 6/8 (75) | 6/6 (100) | .473 | 11/13 (85) | 1/1 (100) | ||
| Therapeutic outcomes | |||||||
| Early treatment failure requiring rescue | 0b | 0b | 0 | 0 | 0 | 0 | |
| Late clinical failure | 2 (1.9) | 0 | 2 (2.5) | 0 | 0 | 0 | |
| Late parasitological failure | 7 (6.8) | 8 (7.8) | 7 (8.9) | 7 (9.0) | 0 | 1 (4.2) | |
| Post-treatment blood-stage P. vivax emergence | 25 (24) | 22 (21.6) | 19 (24.1) | 6 (7.7) | 18 (75) | 4 (16.7) | |
| PCR-adjusted treatment efficacy at 28 d, % | |||||||
| Per protocol | 98.8 [93.5–100] | 100 [95.6–100] | 1 | 98.5 [91.8–100] | 100 [94.3–100] | 100 [80.5–100] | 100 [81.5–100] |
| Intention to treat b | 80.6 [71.6–87.7] | 79.4 [70.3–86.8] | .834 | 82.3 [72.1–90.0] | 80.8 [70.3–88.8] | 70.8 [48.9–87.4] | 75 [53.3–90.2] |
| Cumulative P. falciparum efficacy by K-M analysis c | 98.9 [92.5–99.8] | 100 | .317 | 90.3–99.8 | 100 | 100 | 100 |
| PCR-adjusted treatment efficacy at 42 d, % | |||||||
| Per protocol | 88.7 [79.0–95.0] | 91.4 [82.3–96.8] | .593 | 86.0 [73.8–93.6] | 90.6 [79.34–96.87] | 100 [78.2–100] | 94 [69.8–99.8] |
| Intention to treat b | 62.1 [52.0–71.5] | 63.7 [53.6–73.0] | .814 | 62.0 [50.4–72.7] | 64 [52.44–74.66] | 62.5 [40.6–81.2] | 63 [40.6–81.2] |
| Modified ITT d | 87.4 [79.4–93.1] | 89.2 [81.5–94.5] | .683 | 87.3 [78.0–93.8] | 89.74 [80.8–95.5] | 87.5 [67.6–97.3] | 87.5 [67.6–97.3] |
| Cumulative P. falciparum efficacy by K-M analysis c | 90.4 [81.6–95.1] | 92.0 [82.9–96.3] | .617 | 87.8 [77.1–93.8] | 91.4 [80.5–96.3] | 100 | 93.8 [63.2–99.1] |
Data are median (IQR), n/N (%) [95% CI], or No. (%). Adequate clinical and parasitological response was defined as the absence of parasitemia by the specified end date (28 or 42 days). Per-protocol analysis excluded patients who had withdrawn or were lost to follow-up or who had P.v. recurrence. Late treatment failures were defined as parasitemia between days 4 and 42 and included late clinical failures with symptoms plus parasitemia present and late parasitologic failures with parasitemia only.
Abbreviations: ACPR, adequate clinical and parasitological response; AP, atovaquone-proguanil; ASAP, artesunate-atovaquone-proguanil; CI, confidence interval; IQR, interquartile range; ITT, intention to treat; K-M, Kaplan-Meier; PCR, polymerase chain reaction; PCT1/2, parasite clearance half-life; PCT50, 50% parasite clearance; PCT90, 90% parasite clearance; PCT95, 95% parasite clearance.
aSubject 048 (AP) met the definition for early treatment failure of ongoing parasitemia and fever at 72 hours but was not counted due to concomitant illness explaining fever.
bIntention-to-treat analysis included any patient who had 1 dose of study medication and then withdrew (includes 2 serious adverse events), met a secondary end point (new P. falciparum or P. vivax infection), or was lost to follow-up as they did not complete treatment.
cKaplan-Meier survival analysis included subjects with P.f. only and subjects censored on the day of a newly acquired P.f. infection based on PCR adjustment, P.v. recurrence, loss to follow-up, or withdrawal.
dModified intention to treat where P.v. recurrence was not classified as failure.
eBased on an ex vivo assay of patient plasma activity against the W2 Indochina laboratory P.f. strain [47]. The lower limit of quantification was 17.58 nM DHA equivalents; any participant with activity greater than the lower limit of quantification of DHA activity was deemed positive.
fArtesunate resistant parasite phenotype with parasite clearance time ≥72 hours and parasite clearance half-life of <5 hours.
Safety
AP combined with AS was well tolerated, with only 2 serious adverse events. One ASAP volunteer had asymptomatic obstructive jaundice, with elevated bilirubin, alkaline phosphatase, and aspartate aminotransferase all >4× upper limit of normal by day 7 and normalizing by week 2 with normal alanine aminotransferase throughout. The volunteer suffered no permanent injury, and although a relationship to the study drug was unexpected, it could not be definitively ruled out. One AP volunteer presented with gingival bleeding 1 month after treatment and was diagnosed with idiopathic thrombocytopenic purpura (ITP), which was assessed as unlikely to be related to treatment due to normal blood counts 1 week post-treatment and delayed symptom presentation.
Clinical Outcomes
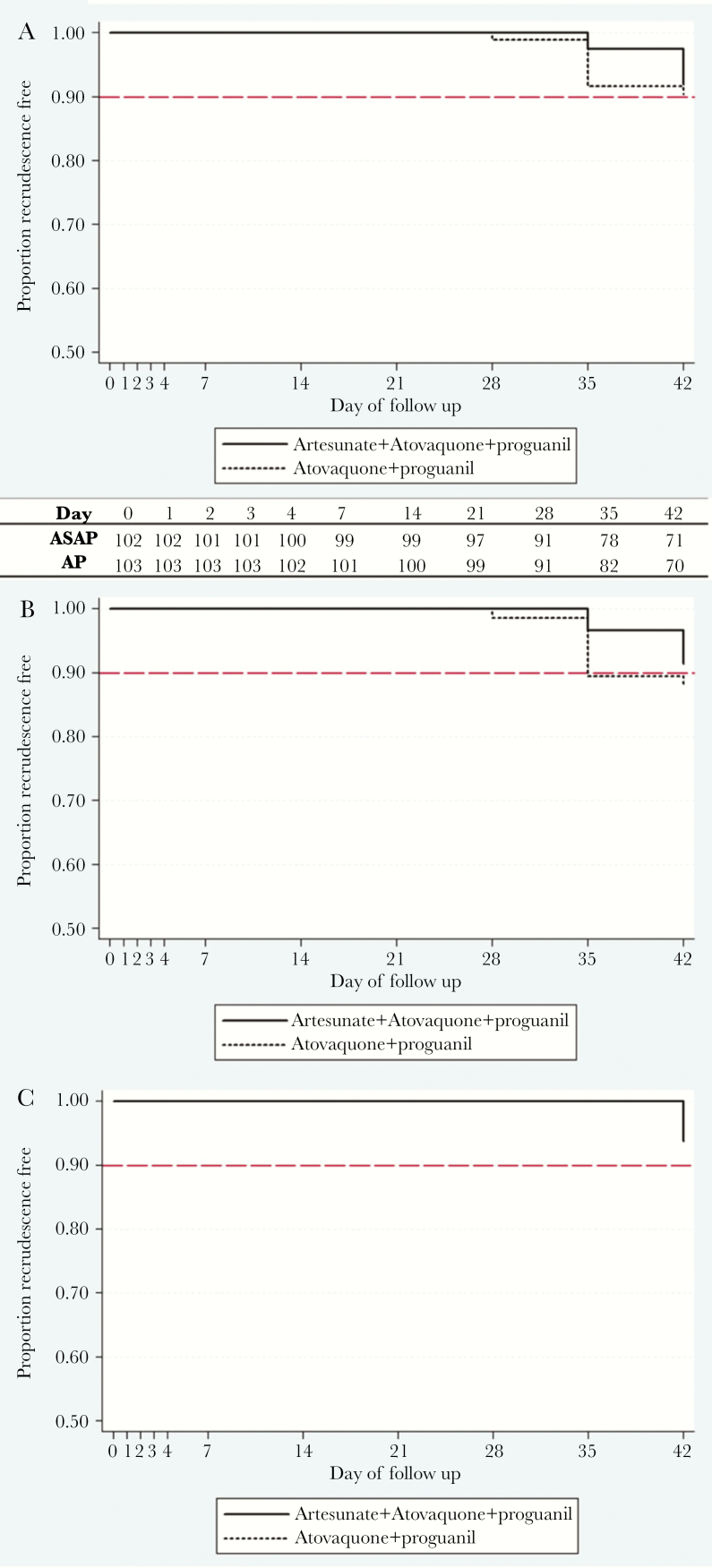
PCR-adjusted ACPR against blood-stage malaria infection was similar for the 2 regimens at 42 days: 90% for AP (95% confidence interval [CI], 82%–95%) vs 92% for ASAP (95% CI, 83%–96%; P = .617 by Kaplan-Meier analysis) (Table 2, Figure 2A–C). There were 17 P.f. malaria recurrences by microscopy, nearly all occurring at the AV site (16/17), with 14 PCR-confirmed recrudescences. Thus, although 28-day per-protocol efficacy was at or near 100% at both sites, 42-day efficacy of both regimens was lower at the AV site (Table 2). Treatment outcomes did not differ by baseline parasitemia. Twenty-five percent of volunteers were excluded from per-protocol analysis during follow-up for receiving blood-stage P. vivax treatment.
Figure 2.
Clinical efficacy. Kaplan-Meier survival analysis of artesunate-atovaquone-proguanil (ASAP) efficacy over 42 days vs atovaquone-proguanil (AP) alone at 2 sites in Cambodia based on number of volunteers remaining malaria free at each interval (inset). A, Overall (log-rank P value for difference = .617). B, Anlong Veng site. C, Kratie site. There were no statistical differences in outcomes for AP (P = .663) or ASAP (P = .317) by location.
Parasite Clearance
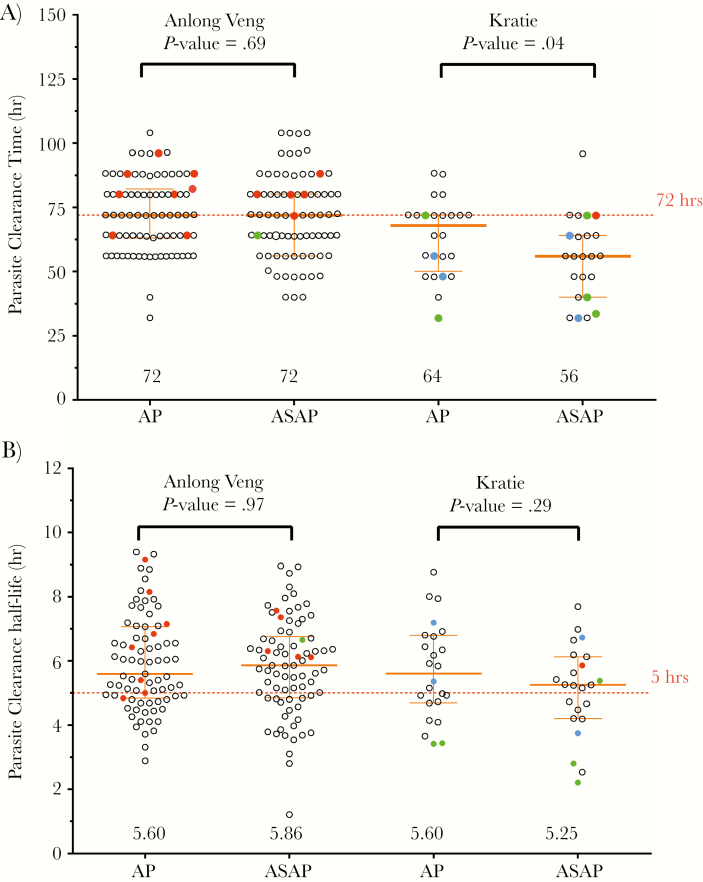
The median parasite clearance time (PCT) in AV was 72 hours for both treatment arms, but was shorter in KT (ASAP 56 hours vs AP 68 hours; P < .001) (Figure 3A, Table 2). At day 3 post-treatment, 42% at the AV site remained microscopy-positive compared with 10% at the KT site (Table 2). However, when measuring parasite clearance half-lives (PCT1/2), there were not significant differences between sites or intervention arms (Figure 3B).
Figure 3.
Parasite clearance times (A) and parasite clearance half-life (B) in Northern (Anlong Veng) and Eastern (Kratie) Cambodia by treatment regimen. All patients with treatment failure assigned to ASAP had a parasite clearance half-life (PCT1/2) >5 hours. Red dots represent parasite recrudescence based on genotyping with msp1, msp2, and glurp polymorphisms. Clear dots represent parasites with the K13 propeller C580Y mutation, green dots represent K13 wild-type parasites, and blue dots represent K13 propeller status unknown. The orange error bars represent medians and interquartile ranges for the variables presented. Comparison of parasite clearance time by t test with Mann-Whitney test revealed significantly faster parasite clearance time for the ASAP treatment vs AP in Eastern Cambodia only (P = .04). There were no significant differences in parasite clearance half-life overall. The median PCT1/2 was significantly higher at 5.34 nM for parasites with the K13 propeller C580Y mutation vs 3.42 for parasites without C580Y (P = .0032).
The majority of treatment failures (12/14) occurred in patients infected with artemisinin-resistant phenotype parasites with PCT >72 hours and PCT1/2 >5 hours (Table 2, Figure 3). Despite faster parasite clearance and fewer treatment failures in Kratie, nearly all isolates at both sites carried the C580Y K13 mutation (AV 99% [168/169] vs KT 90% [38/43]) (Figure 3), with the remaining isolates having K13 wild-type. All treatment failures were C580Y positive, with C580Y parasites cleared more slowly than non-C580Y parasites (median PCT1/2, 5.3 in C580Y vs 3.4 hours in non-C580Y infections; P = .003).
Parasite Drug Sensitivity
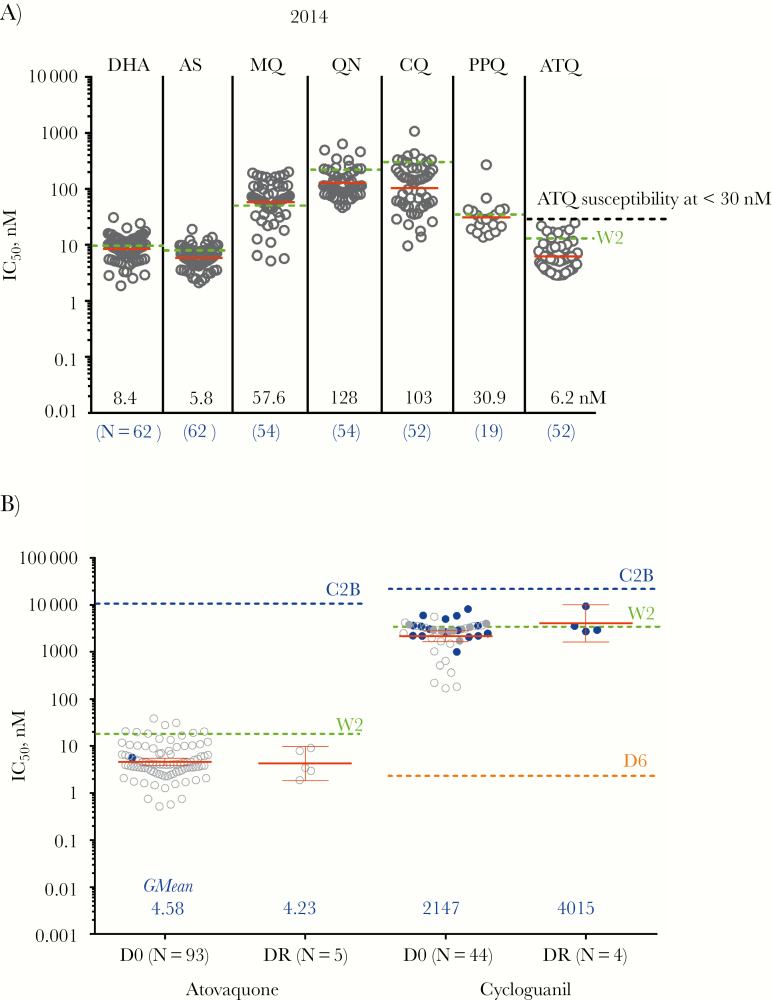
Fresh patient P.f. isolates retained comparable resistance patterns to recent studies at the Anlong Veng site by HRP2 ELISA (Figure 4A) [4]. Notably, isolates retained ex vivo atovaquone sensitivity at both sites, with geometric mean pretreatment IC50 of 4.8 nM (95% CI, 3.8–6.0) in AV and 3.5 nM (95% CI, 2.4–5.0) in KT (P = .02). ATQ IC50 was evaluable in 5/14 recrudescent samples (36%), with a geometric mean IC50 similar to baseline (4.2 nM; P = .51). All isolate IC50 were far below that of the ATQ-resistant C2B clone (11 368 nM) (Figure 4B) [22]. Sanger sequencing did not detect a mutation at cytb Y268 associated with ATQ resistance in any baseline isolates. Only 1 volunteer (ASAP-90) harbored the Y268C mutation at recrudescence (1/14), though IC50 was unevaluable. Sequencing across the entire mitochondrial cytochrome b gene was successful in 14 recrudescent isolates, but no previously reported [23–25] or new single nucleotide polymorphisms (SNPs) were found.
Figure 4.
Immediate ex vivo Plasmodium falciparum (P.f.) parasite resistance. A, Overall ex vivo P.f. parasite sensitivity to commonly used antimalarial drugs in 2014 from field surveillance stations in Cambodia. Red bars represent geometric mean values, whereas green dashed lines represent values for the W2 Indochina P.f. clone. B. Ex vivo parasite sensitivity by histidine-rich protein 2 enzyme-linked immunosorbent assay to atovaquone and cycloguanil for evaluable isolates on the day of study screening before treatment (D0) and day of recrudescence (DR). Baseline assays were interpretable for only 93 of 202 isolates for atovaquone (46%) and 46 of 160 (29%) for cycloguanil. Clear dots represent parasites with the cytb atovaquone resistance gene wild-type, and blue dots represent K13 propeller status unknown. Red bars in each column represent geometric mean 50% inhibitory concentration (IC50) values, whereas dashed green lines represent mean values for the chloroquine-resistant W2 Indochina clone run simultaneously for each assay. Dashed blue lines represent geometric mean values for the atovaquone-resistant C2B clone. Dashed orange lines represent geometric mean values for the chloroquine-sensitive D6 clone. Geometric mean atovaquone IC50 for parasites with the C580Y mutation was significantly higher (4.57 nM; n = 94) than for non-C580Y parasites (1.29 nM; n = 4; P = .001).
Parasites displayed high-level cycloguanil (CYC) resistance (geometric mean pretreatment IC50, 2204 nM), though only 29% of isolates were successfully evaluated ex vivo (Figure 4B). No cycloguanil-specific dihydrofolate-reductase (DHFR) mutations (S108T and A16V) were detected in any of the tested isolates. All 14 recrudescent samples had 4 parasite DHFR gene SNPs (S108N, N51II, C59R, and I164L) associated with pyrimethamine resistance and were thought to confer cycloguanil cross-resistance [26, 27]. A randomly sampled subset of 46 baseline AV samples were also quadruple pyrimethamine mutants, consistent with fixation of this DHFR genotype.
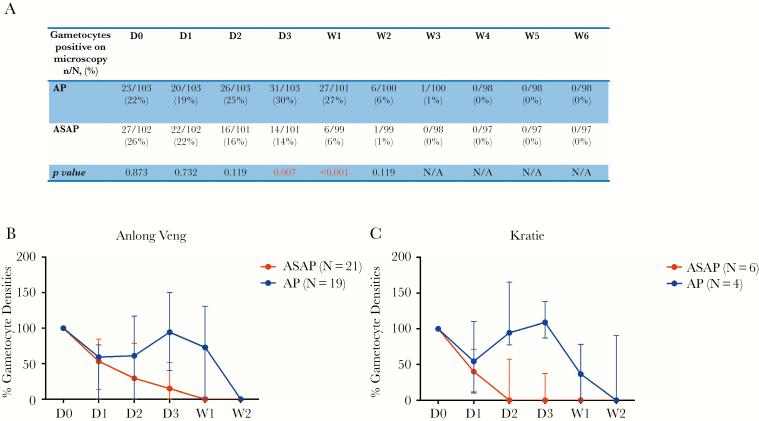
Gametocyte Carriage After Treatment
At screening, 22% of volunteers treated with AP had gametocytemia by microscopy vs 26% for ASAP (n.s.) (Figure 5A). ASAP-treated volunteers had faster gametocyte decline than AP, reaching statistical significance by day 3 (P = .0009) and remaining significantly lower in the ASAP arm through week 2, despite universal baseline 15-mg primaquine treatment (Figure 5). Median gametocyte densities normalized for baseline values revealed more rapid clearance for the ASAP group (Figure 5B and C).
Figure 5.
Gametocyte carriage by light microscopy. A, Number and percentage of subjects found to have Plasmodium falciparum gametocytes by light microscopy from each treatment arm during 6 weeks of follow-up. B and C, Median parasite gametocyte densities over the first 2 weeks of follow-up for those subjects who were originally gametocytemic, normalized to baseline values on D0 (100%).
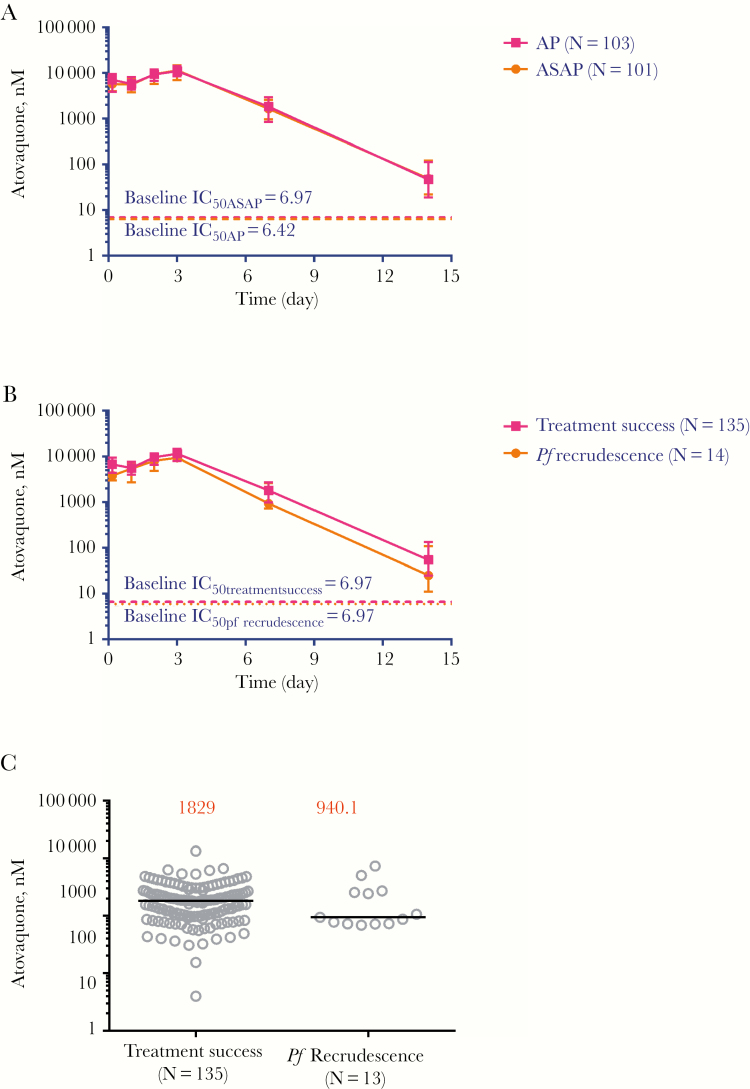
Pharmacology
Mean atovaquone levels were not significantly different between treatment groups (Figure 6A) or treatment outcomes (Figure 6B). Mean atovaquone levels on day 7 in those with ACPR were not different than in those who recrudesced (Figure 6C).
Figure 6.
Atovaquone levels after drug administration. A, Atovaquone levels in nanomoles (nM) per milliliter (mL) of plasma in patients with uncomplicated malaria dosed with atovaquone-proguanil (AP) or artesunate-atovaquone-proguanil (ASAP) for 3 days. Horizontal lines represent mean parasite atovaquone 50% inhibitory concentration (IC50) values in nM at baseline for the respective treatment groups. Dashed horizontal lines represent mean parasite atovaquone IC50 values at baseline for the respective study groups. B, Atovaquone levels in patients who were cured (ACPR) and those who had a malaria recurrence. Horizontal lines represent mean parasite atovaquone IC50 values in nM at baseline for the respective groups. C, Median day 7 drug levels of atovaquone for ACPR and recrudescent patients. One patient in the recrudescence group missed follow-up on day 7.
Discussion
Implications for Implementation of Rescue Therapies in Areas of Severe Multidrug Resistance
Combining a 3-day course of AP with AS in a large, rigorous study was shown in 2004 to improve efficacy against MDR P.f. to near 100% in Thailand [13]. A decade later in Cambodia, both regimens approached 100% 28-day PCR-corrected efficacy, but hovered at 90% by day 42. Findings here are consistent with previous observations that AP treatment failures before day 28 are rare [28] and thought to be due to poor drug absorption. AP was administered with food under the study protocol to ensure sufficient absorption. Low dropout rates (<5%) lend confidence to the efficacy findings.
Given recent failures of firstline treatment drugs [1, 7], it was hoped that the ASAP combination might preserve drug efficacy. There has been a measurable shift in antimalarial resistance patterns in the past 17 years since van Vugt et al. published their original report in 2002 [13]. At that time, artemisinin resistance had yet to be clearly defined. Atovaquone-proguanil had not yet been deployed in Western Cambodia to mitigate emerging resistance, and it was 12 years before clinical dihyrdroartemisinin-piperaquine failures were reported in Cambodia. Recent claims of malaria “superbugs” in Cambodia and elsewhere [29] underscore the urgent need for alternative regimens using therapies in hand, as was attempted in the present trial. We sought to determine whether AP remains a deployable option and whether AS might help to boost its efficacy. Given the resultant 90%–92% efficacy estimate here compared with 97%–99% previously observed in neighboring Thailand, there is reason to believe this approach would be suboptimal in Cambodia. High rates of microscopic gametocytemia, even in combination with low-dose primaquine, also raise concerns regarding the utility of ASAP as a firstline regimen in an elimination campaign.
Atovaquone Resistance
Despite a nearly 10% treatment failure rate, measurable atovaquone resistance was not observed, with adequate drug levels well above baseline parasite IC50 and absent cytochrome bc1 mutations in all but 1 volunteer detected at week 5. Interestingly, this single mutation was not detected at baseline, suggesting that it may have arisen de novo or existed as a minority variant that was selected through treatment. De novo mutations refer to cytb Y268 found at recrudescence, but not at baseline, before treatment. Despite rare de novo mutations, AP resistance appears to be an all-or-none rather than gradual phenomenon [30]. In prior studies, most clinical failures attributable to cytb mutations occurred beyond 28 days. However, only 1 failure here among 14 was potentially attributable to cytb mutation. This was unexpected and argues against atovaquone resistance as the cause of treatment failures. As genotyping was performed here, based on PCR amplification and Sanger sequencing (capturing the majority genotype), this does not preclude that cytb Y268 mutations might have been present as minority variants at baseline. Preserved ex vivo atovaquone sensitivity was found among isolates from a 2013 clinical study at the AV site despite >50% clinical dihydroartemisinin-piperaquine failure rates [10], and again here 2 years later. Atovaquone-proguanil was used in WHO-sponsored malaria containment efforts along the Thai border beginning in 2006 [9], making the lack of apparent atovaquone resistance surprising. This could be due to apparent loss of parasite fitness in the presence of cytb mutations, with parasites developing cytb mutations being less likely to persist in the population [31].
Short-Acting Agent Resistance
Insufficient short-acting blood-stage agent activity may have contributed to reduced clinical efficacy. In vitro cycloguanil IC50 were elevated in the presence of PfDHFR mutations, which were previously associated with pyrimethamine resistance and thought to be associated with cycloguanil cross-resistance [28]. This suggests, though it does not confirm, that cycloguanil resistance may have played a role in the clinical failures seen here. Although parasites assayed displayed high levels of cycloguanil resistance, this finding is tempered by the fact that less than one-third of isolates yielded interpretable ex vivo cycloquanil sensitivity results. The lack of cycloguanil-specific dihydrofolate-reductase (DHFR) mutations (S108T and A16V) in any of the isolates tested further calls potential resistance into question. The strongest evidence appears to be the presence of 4 parasite DHFR gene SNPs (S108N, N51II, C59R, and I164L) in all of the 14 recrudescent samples. Although associated with pyrimethamine resistance, prior work has indicated that they may confer cycloguanil cross-resistance [26, 27]. These quadruple pyrimethamine mutants were found to be at fixation for this DHFR genotype. This suggests high-level DHFR resistance, likely due to persistant resistance genotypes from parasites exposed to antimalarials and other drugs operating through the DHFR mechanism. The use of trimethoprim-sulfamethoxazole and other DHFR inihibitors as antibiotics is common in the region and has led to high levels of bacterial resistance [32].
Unlike the prior study in Thailand [13], there was no added benefit of AS on blood-stage efficacy or PCT1/2. It was previously suggested that AS should be added to AP well before AP resistance develops, but in the present study longstanding AS resistance may have rendered this issue moot. The C580Y K13 artemisinin-resistance mutation approached fixation, suggesting contributions of both cycloguanil and artesunate resistance (ART-R) to declining efficacy. The majority of clinical failures for both regimens occurred at the Along Veng site, where longstanding ART-R has contributed to rapidly developing artemisinin combination therapy (ACT) failures [1, 33]. AP had not been used, nor has significant ART-R been previously reported, at the Kratie site. Blood-stage ASAP efficacy was well preserved despite high rates of K13 C580Y in Kratie (>85%), supporting previous evidence that additional mutations may be needed to confer clinically apparent ART-R [34]. Unfortunately, there is only limited evidence here that artesunate and/or cycloguanil resistance genotypes may have contributed to the modest decline in efficacy. Study design precludes distinguishing the relative contributions of each.
Implications for the Addition of Low-Dose Primaquine to AP-Containing Regimens
To date, primaquine has been the only clinically available drug shown to effectively kill mature P.f. gametocytes, preventing ongoing transmission [35]. Current WHO guidelines for treatment of P.f. malaria recommend single low-dose primaquine with blood-stage ACT treatment, particularly in low-transmission areas where untreated gametocyte carriers maintain transmission [36]. A recent meta-analysis concluded that malaria control is limited by poor estimation of the gametocyte reservoir, with significant underestimates by light microscopy [37]. However, higher transmission risk has been demonstrated for microscopically patent infections [38]. Pre- and post-treatment microscopic gametocytemia were higher in both treatment arms here compared with a prior study at the same site [39] that used dihydroartemisinin-piperaquine as the blood-stage agent [1, 16] along with a higher 45-mg dose of primaquine (PQ). Though clinical data remain sparse, lower post-treatment gametocytemia seen in the ASAP arm was similar to previous findings in Thailand [13], suggesting added benefit of AS to eliminate sexual-stage parasites, and presumably reduce transmission. Lack of a PQ control arm here limits conclusions regarding the efficacy of low-dose PQ to treat gametocytes. Despite this limitation, the data suggest both higher baseline microscopic gametocytemia and lower sexual-stage efficacy than previously observed at the Anlong Veng site. In a 2013 randomized trial, pre-enrollment microscopic gametocytemia was roughly 10%, whereas the number carrying microscopically detectable gametocytemia at 1 week was significantly lower in those randomized to 45-mg single-dose PQ compared with untreated patients [16]. Evaluating potential drug–drug interactions between AP and PQ as a possible cause of higher-than-expected post-treatment gametocytemia may be useful.
Despite earlier in vitro and animal evidence, AP does not appear to have been an effective transmission-blocking agent on its own. High rates of microscopic gametocytemia despite single low-dose primaquine administration were seen here, with 30% still positive at day 3, suggesting increased malaria transmission risk [38]. Although concerning, the clinical significance of apparently increased gametocytemia on transmission of atovaquone-resistant malaria remains uncertain. Atovaquone-resistant P. berghei parasites were not transmissible to mosquitoes in animal models [28] and had reduced P.f. gametocytemia in vitro [40]. Rapid in-host propagation of Y268S cytb atovaquone resistance mutation has also been attributed to mitochondrial heteroplasmy [29]. Heteroplasmy may lead to clinical resistance in the presence of lower IC50 values than previously proposed for clinical resistance (29 nM) [28].
Implications for P. vivax Treatment and Malaria Elimination
High rates of post-treatment blood-stage P.v. were consistent with limited evidence of AP’s poor efficacy against blood-stage [28] and tissue-stage P.v. [41]. Concerns over rapid development of blood-stage Pf resistance and cost have limited AP use to high-risk areas in Southeast Asia. Mathematical modeling suggests that it may be a poor option for elimination if used alone [42]. Although AP remains a rational choice for rescue therapy in Cambodia, its utility as a firstline agent outside areas of severe multidrug resistance remains unclear. In a recent report by the WHO, to delay resistance onset it was recommended that AP be tested in combination with ACTs [43]. Although use of AP in combination with ACTs had been suggested as a treatment for MDR P.f., it requires consideration of potential of drug–drug interactions and evidence of improved efficacy [44]. There may also be implications for the use of AP as a chemoprophylaxis agent. However, these are less clear, as AP is thought to act primarily in the liver on pre-erythrocytic parasite stages (sometimes referred to as causal activity) [11]. To date, there have been only limited reports of AP failures when used as chemoprophylaxis. The recent approval of tafenoquine for both the radical cure of P. vivax and antimalarial chemoprophylaxis offers a compelling new alternative where screening for G6PD deficiency exists [45].
Study Limitations
Although there is an insufficient number of samples to make strong conclusions about mutation prevalence, the results provide useful information to develop a greater understanding of AP resistance in the setting of ART-R. In an area with no discernable atovaquone resistance mutations or other clear explanations for failure rates, AP efficacy is declining nonetheless. This finding is challenging to explain given near universal K13 C580Y ART-R mutations but negligible rates of cytochrome bc1 mutations conferring ATQ resistance. The population studied was overwhelmingly male. Although this may compromise the generalizability of results to women and children to some extent, malaria risk in Cambodia is largely occupational. With men making up the bulk of security and forestry workers in these at-risk professions, this may be an accurate representation of overall malaria risk. Another important limitation is an imbalance in sample size between KT and AV that proved unavoidable. AP efficacy was higher at the Kratie site, despite a lack of differences in bc1 mutations, though the study was not powered to detect site-specific differences. Lower enrollment in Kratie limits definitive conclusions on both asexual- and sexual-stage efficacy by site. The sample size difference was governed as much by logistical as scientific considerations and is one of the challenges of executing clinical trials in austere settings. The AV site was well established before study inception, but several months were spent getting the KT site online after study initiation. Establishing qualified sites capable of carrying out well-regulated clinical trials in remote areas is essential for conducting effective and informative clinical research in this region. Although definitive proof of different resistance patterns between the two sites cannot be established in the present study, important qualitative differences were observed, and the resistance patterns were not unexpected. From a statistical standpoint, the study was intended to define differences in effectiveness of the 2 regimens across both sites.
Conclusions
Given recent treatment failures, there’s been considerable debate on optimal treatment of MDR malaria in the region. Although not yet at “untreatable malaria,” optimizing use of available therapies and placing greater emphasis on nonpharmacologic approaches to malaria control should be employed in tandem [7]. Although clinical AP activity appears preserved in Eastern Cambodia, it is clearly under pressure in Northwestern Cambodia, with cycloguanil resistance being a possible contributor. AP remains a reasonable rescue drug but cannot be recommended as a firstline agent in Cambodia. Co-administration of artesunate may reduce post-treatment gametocyte carriage, despite little added blood-stage efficacy. Single low-dose primaquine administered with AP for this purpose appeared relatively ineffective compared with other blood-stage regimens [46] and higher doses of primaquine [16]. Given the findings, nonpharmacologic approaches to containing antimalarial resistance in Cambodia should be pursued.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank Dr. Krisada Jongsakul for his leadership, support and dedication to the study. The authors would also like to acknowledge Dr. Charlotte Lanteri, Ms. Emily Cisney, Dr. Jessica Manning, Dr. Ilin Chuang, Dr. Carrie Gregory, Mr. Mok Rangseay, Dr. Paktiya Teja-isavadharm, Dr. Sathit Pickyangkul and Ms. Nitima Chanarat. Above all, we would like to thank the volunteers and their families who participated in the study, and the staff of the Anlong Veng and Kratie Referal Hospitals and Provincial Health Departments. We would finally like to thank the Royal Cambodian Armed Forces for their steadfast support of the study throughout.
Financial support. This work was supported by the Naval Advanced Medical Development Program, Washington, DC. The funding source had no role in the analysis or interpretation of data, preparation of the manuscript or the decision to publish.
Disclaimer. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Potential conflicts of interest. The authors have no conflicts of interest to declare. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Conceptualization: D.S., C.L., J.T.L., D.H., A.R., L.D., S.P. Data curation: C.L., A.R., B.C., S.C., S.Ch., M.I., N.B., T.B., W.K., K.T., K.N., P.G., J.T.L., P.V., M.W. Formal analysis: J.T.L., T.B., S.C., S.Ch., M.I., N.B., W.K., P.V., P.G., M.W., C.L., D.S. Funding acquisition: D.S., D.H., J.T.L., C.L., L.D. Investigation: D.S., C.L., S.S., J.T.L., D.H., C.B., M.W., K.J., S.C., S.Ch., M.I., N.B., M.D.S., W.K., K.T., K.N., V.H., P.V., P.G., L.D. Methodology: D.S., C.L., J.T.L., W.K., A.R., D.H., S.Ch., V.H., T.K.H., K.N., K.J., S.C., N.B., M.I. Project administration: D.S., A.V., D.H., J.T.L., C.L., M.W., C.B., S.C., M.I., W.K., K.T., K.J., A.R., V.H., P.G., R.U., M.M.F., P.S., L.D., S.S., S.P. Supervision: D.S., A.V., D.H., C.B., J.T.L., C.L., M.W., P.S., M.M.F., K.J., L.D. Validation: T.B., A.R., B.C., J.T.L., S.C., N.B., M.I., W.K., K.T., P.G. Visualization: P.V., J.T.L., M.W., A.R., T.B., P.G., D.S. Writing ± original draft: D.S., M.W., J.T.L., C.L., P.V., P.G., H.B., T.B. Writing ± review & editing: D.S., M.W., J.T.L., C.L., P.V., P.G., H.B., T.B., M.D.S.
References
- 1. Spring MD, Lin JT, Manning JE, et al. . Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis 2015; 15:683–91. [DOI] [PubMed] [Google Scholar]
- 2. Amaratunga C, Lim P, Suon S, et al. . Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 2016; 16:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kingdom of Cambodia. National Treatment Guidelines for Malaria in the Kingdom of Cambodia. Phnom Penh, Cambodia: Kingdom of Cambodia; 2012. [Google Scholar]
- 4. Chaorattanakawee S, Saunders DL, Sea D, et al. . Ex vivo drug susceptibility testing and molecular profiling of clinical Plasmodium falciparum isolates from Cambodia from 2008 to 2013 suggest emerging piperaquine resistance. Antimicrob Agents Chemother 2015; 59:4631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parobek CM, Parr JB, Brazeau NF, et al. . Partner-drug resistance and population substructuring of artemisinin-resistant Plasmodium falciparum in Cambodia. Genome Biol Evol 2017; 9:1673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amato R, Lim P, Miotto O, et al. . Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis 2017; 17:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saunders D, Lon C. Combination therapies for malaria are failing—what next? Lancet Infect Dis 2016; 16:274–5. [DOI] [PubMed] [Google Scholar]
- 8. Manning JE, Satharath P, Gaywee J, et al. . Fighting the good fight: the role of militaries in malaria elimination in Southeast Asia. Trends Parasitol 2014; 30:571–81. [DOI] [PubMed] [Google Scholar]
- 9. Hoyer S, Nguon S, Kim S, et al. . Focused Screening and Treatment (FSAT): a PCR-based strategy to detect malaria parasite carriers and contain drug resistant P. falciparum, Pailin, Cambodia. PLoS One 2012; 7:e45797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saunders DL, Chaorattanakawee S, Gosi P, et al. . Atovaquone-proguanil remains a potential stopgap therapy for multidrug-resistant Plasmodium falciparum in areas along the Thai-Cambodian border. Antimicrob Agents Chemother 2015; 60:1896–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg 2007; 76:208–23. [PubMed] [Google Scholar]
- 12. Wichmann O, Muehlen M, Gruss H, et al. . Malarone treatment failure not associated with previously described mutations in the cytochrome b gene. Malar J 2004; 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Vugt M, Leonardi E, Phaipun L, et al. . Treatment of uncomplicated multidrug-resistant falciparum malaria with artesunate-atovaquone-proguanil. Clin Infect Dis 2002; 35:1498–504. [DOI] [PubMed] [Google Scholar]
- 14. US Pharmacopeia. Uniformity of dosage units (905) Available at: https://www.usp.org/harmonization-standards/pdg/general-methods/uniformity-dosage-units Accessed 1 December 2011.
- 15. Bethell D, Se Y, Lon C, et al. . Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS One 2011; 6:e19283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin JT, Lon C, Spring MD, et al. . Single dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission in Cambodia: an open-label randomized trial. PLoS One 2017; 12:e0168702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rougemont M, Van Saanen M, Sahli R, et al. . Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol 2004; 42:5636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03 2010. Available at: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 10 October 2017.
- 19. Gosi P, Lanteri CA, Tyner SD, et al. . Evaluation of parasite subpopulations and genetic diversity of the msp1, msp2 and glurp genes during and following artesunate monotherapy treatment of Plasmodium falciparum malaria in Western Cambodia. Malar J 2013; 12:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor SM, Parobek CM, Aragam N, et al. . Pooled deep sequencing of Plasmodium falciparum isolates: an efficient and scalable tool to quantify prevailing malaria drug-resistance genotypes. J Infect Dis 2013; 208:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rutvisuttinunt W, Chaorattanakawee S, Tyner SD, et al. . Optimizing the HRP-2 in vitro malaria drug susceptibility assay using a reference clone to improve comparisons of Plasmodium falciparum field isolates. Malar J 2012; 11:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fivelman QL, Adagu IS, Warhurst DC. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob Agents Chemother 2004; 48:4097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talundzic E, Plucinski MM, Biliya S, et al. . Advanced molecular detection of malarone resistance. Antimicrob Agents Chemother 2016; 60:3821–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plucinski MM, Huber CS, Akinyi S, et al. . Novel mutation in cytochrome b of Plasmodium falciparum in one of two atovaquone-proguanil treatment failures in travelers returning from same site in Nigeria. Open Forum Infect Dis 2014; XXX(X):XXX –XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korsinczky M, Chen N, Kotecka B, et al. . Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother 2000; 44:2100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mharakurwa S, Kumwenda T, Mkulama MA, et al. . Malaria antifolate resistance with contrasting Plasmodium falciparum dihydrofolate reductase (DHFR) polymorphisms in humans and Anopheles mosquitoes. Proc Natl Acad Sci U S A 2011; 108:18796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis 2002; 2:209–18. [DOI] [PubMed] [Google Scholar]
- 28. Staines HM, Burrow R, Teo BH, et al. . Clinical implications of Plasmodium resistance to atovaquone/proguanil: a systematic review and meta-analysis. J Antimicrob Chemother 2018; 73:581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arie S. Researchers and WHO clash over global threat of drug resistant malaria. BMJ 2017; 359:j5127. [DOI] [PubMed] [Google Scholar]
- 30. White NJ. Preventing antimalarial drug resistance through combinations. Drug Resist Updat 1998; 1:3–9. [DOI] [PubMed] [Google Scholar]
- 31. Goodman CD, Siregar JE, Mollard V, et al. . Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science 2016; 352:349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng CY, Smith BL, Bodhidatta L, et al. . Etiology of diarrhea in young children and patterns of antibiotic resistance in Cambodia. Pediatr Infect Dis J 2011; 30:331–5. [DOI] [PubMed] [Google Scholar]
- 33. Saunders DL, Vanachayangkul P, Lon C; US Army Military Malaria Research Program; National Center for Parasitology, Entomology, and Malaria Control (CNM); Royal Cambodian Armed Forces Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 2014; 371:484–5. [DOI] [PubMed] [Google Scholar]
- 34. Miotto O, Amato R, Ashley EA, et al. . Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet 2015; 47:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashley EA, Recht J, White NJ. Primaquine: the risks and the benefits. Malar J 2014; 13:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Updated WHO Policy Recommendation (October 2012): single dose Primaquine as a gametocytocide in Plasmodium falciparum malaria Available at: http://www.who.int/malaria/pq_updated_policy_recommendation_en_102012.pdf. Accessed 20 May 2016.
- 37. Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 2009; 200:1509–17. [DOI] [PubMed] [Google Scholar]
- 38. Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol 2014; 30:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin JT, Ubalee R, Lon C, et al. . Microscopic Plasmodium falciparum gametocytemia and infectivity to mosquitoes in Cambodia. J Infect Dis 2016; 213:1491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fleck SL, Pudney M, Sinden RE. The effect of atovaquone (566C80) on the maturation and viability of Plasmodium falciparum gametocytes in vitro. Trans R Soc Trop Med Hyg 1996; 90:309–12. [DOI] [PubMed] [Google Scholar]
- 41. Povinelli L, Monson TA, Fox BC, et al. . Plasmodium vivax malaria in spite of atovaquone/proguanil (malarone) prophylaxis. J Travel Med 2003; 10:353–5. [DOI] [PubMed] [Google Scholar]
- 42. Maude RJ, Nguon C, Dondorp AM, et al. . The diminishing returns of atovaquone-proguanil for elimination of Plasmodium falciparum malaria: modelling mass drug administration and treatment. Malar J 2014; 13:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Global Malaria Program. Reports of the Technical Expert Group on Drug Efficacy and Response Geneva, Switzerland: World Health Organization;2017.
- 44. Schwartz E, Lachish T. Artemisinin-based combination therapy (ACT) versus atovaquone-proguanil: do not choose between but, rather, combine them. Evid Based Med 2016; 21:64. [DOI] [PubMed] [Google Scholar]
- 45. Lacerda MVG, Llanos-Cuentas A, Krudsood S, et al. . Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med 2019; 380:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eziefula AC, Bousema T, Yeung S, et al. . Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis 2014; 14:130–9. [DOI] [PubMed] [Google Scholar]
- 47. Teja-Isavadharm P, Peggins JO, Brewer TG, et al. . Plasmodium falciparum-based bioassay for measurement of artemisinin derivatives in plasma or serum. Antimicrob Agents Chemother 2004; 48:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.