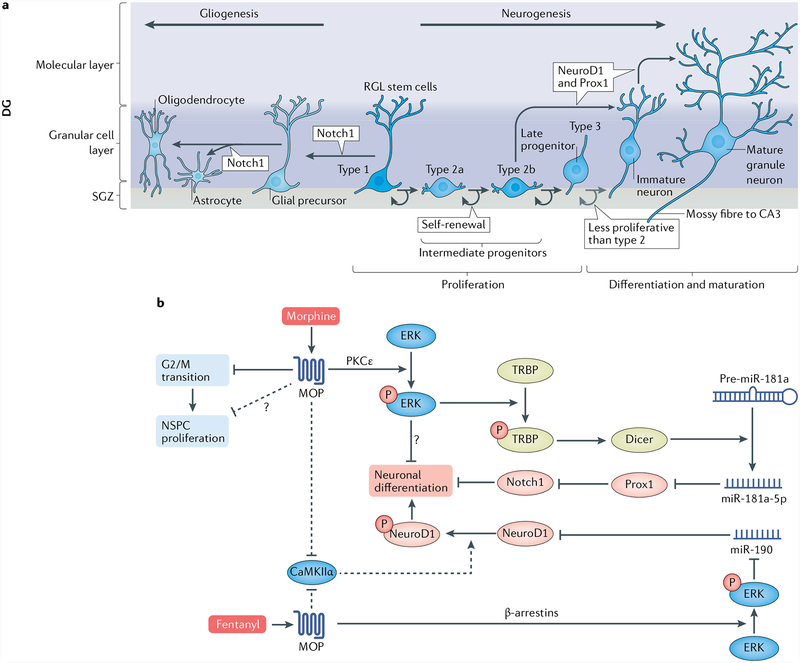
Fig. 3 |. Neurogenesis and biased MOP agonism in NSPC proliferation and differentiation.
a | The subgranuίar zone (SGZ) of the dentate gyrus (DG) provides a unique microenvironment where adult neural stem and progenitor cells (NSPCs) undergo several consecutive developmental stages characterized by different cell types17. NSPCs are derived from embryonic stem cells that survived in the adult SGZ. NSPCs self-renew and include type 1 radiaί-gίia-ίike (RGL) stem cells, which give rise to intermediate progenitor cells type 2a and 2b. These type 2 cells can generate type 3 progenitors (neuroblasts), which are capable of differentiating into immature neurons. During maturation, immature neurons become granule neurons and are functionally integrated into the DG pre-existing circuit. Type 2b and type 3 cells, immature and mature neurons, express the transcription factors NeuroD1 and Prox1, which are specific to granule cell development32. Notch 1 promotes astroglial but not neuronal differentiation17. b | μ-Opioid peptide receptor (MOP) agonists (such as morphine) inhibit NSPC proliferation mainly by slowing the cell cycle G2/M phase transition (prolonged S phase) in mouse embryonic NSPCs28, though details regarding the mechanism remain elusive. Other pathways leading to stagnating proliferation (indicated by the dashed arrow and question mark) might exist. Morphine induces extraceίίuίar-signaί-reguίated kinase (ERK) phosphorylation in a PKCε-dependent manner, and the activated ERK in turn phosphorylates TRBP, which is a stabilizer of Dicer, the essential enzyme for microRNA maturation43. The mature miR-181a-5p then inhibits the transcription factor Prox1, an inhibitor of another transcription factor, Notch1. Thus, increased Notch1 expression mediates the inhibition of neuronal differentiation15. The inhibition of neurogenic differentiation 1 (NeuroD1) phosphorylation is another pathway by which morphine affects the transition of NSPCs into immature neurons in adult mice16. It is inferred that although both morphine and fentanyl affect NeuroD1 phosphorylation by inhibiting CaMKIIα, a crucial enzyme that catalyses NeuroD1 phosphorylation, fentanyl was able to activate NeuroD1 via the β-arrestin-ERK-miR-190 pathway, thus reflecting the biased antagonism of MOP agonists44. Involvement of this pathway has been demonstrated only in cultured primary neurons74.