Abstract
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide expressed both centrally and peripherally. CGRP has been shown to be involved in arteriolar dilation, cardiovascular regulation, pain transmission, migraine, and gastrointestinal physiology. Our current research is aimed at analyzing CGRP’s impact on appetite/satiety, body metabolism, and energy homeostasis. Our study investigated the effects of a single-dose intraperitoneal (IP) treatment with CGRP on food and water consumption, energy expenditure, physical activity, respirometry, and a panel of plasma metabolic hormones in C57Bl/6 wild-type (WT) mice. After a CGRP IP injection at a dose of 2 nmol (10 μMCGRP in 200 μl of saline), a significant reduction in food intake and metabolic parameters as RQ, VCO2, and VO2 was observed. CGRP-injected mice had also significantly lower total energy expenditure (TEE) with no changes in activity levels compared to vehicle-injected controls. CGRP treatment in mice induced significantly lower plasma levels of glucagon and leptin but higher levels of amylin. Our data show that a single dose of CGRP peptide significantly decreased food consumption and altered calorimetric parameters and plasma metabolic hormone levels, thus confirming that CGRP plays a pivotal role in the regulation of appetite and metabolism. Future studies are necessary to analyze CGRP’s long-term impact on body metabolism and its potential effects on appetite, obesity, and metabolic disorders.
Keywords: Calcitonin gene-related peptide, Metabolism, Appetite, Metabolic hormones
Introduction
Obesity is a worldwide epidemic associated with increased morbidity and early mortality. In the USA, over 93 million people are obese, and each year, approximately 300,000 adults die from obesity-associated causes (CDC 2018). The regulation of appetite and feeding behavior, essential to maintaining caloric intake, energy balance, and glucose/body homeostasis, involves both central and peripheral neurohormonal mechanisms whose regulatory pathways are not fully elucidated (Woods et al. 1998). In the central nervous system (CNS), the hypothalamus and brainstem modulate appetite and food intake through both the release of neuropeptides and through feedback signals from the peripheral autonomic nervous system (ANS) (Russell et al. 2014). Peripherally, the mucosa of the gastrointestinal (GI) tract senses nutrients in the lumen and responds by releasing peptides to regulate secretion, motility, and absorption (Greenwood et al. 2011; Hansen 2003).
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide localized to chromosome 11 in humans (Cottrell et al. 2005; Russell et al. 2014). CGRP has been shown to be involved in arteriolar dilation, cardiovascular regulation (Cottrell et al. 2005; Russell et al. 2014), pain transmission (Yarwood et al. 2017), and migraine formation (Kaiser and Russo 2013). CGRP belongs to the hormone superfamily of peptides that includes adrenomedullin (AM), intermedin (adrenomedullin 2) (IMD), and amylin (Russell et al. 2014). CGRP preferentially binds to the heterodimer of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP 1). Binding initiates a G protein-coupled receptor signal transduction cascade (Cottrell et al. 2005; Lennerz et al. 2008; Parsons and Seybold 1997).
CGRP expression has been found near CLR receptors, thus suggesting that this peptide serves as a ligand for the CLR-RAMP1 heterodimers (Cottrell et al. 2005). Specifically, CGRP and CLR-RAMP1 have been found centrally in the trigemino-vascular system, and peripherally in dorsal root ganglia (Cottrell et al. 2005; Lennerz et al. 2008; Parsons and Seybold 1997), as well as in stomach, ileum, and colonic endothelium (Wimalawansa 1990; Wimalawansa 1996). In the stomach, CGRP receptors have been localized in the myenteric plexus and in the intestinal nerve fibers of circular and longitudinal muscles (Cottrell et al. 2005; Kawashima et al. 2002). CGRP has been showed to be released following stimulation of enteroendocrine cells (Wimalawansa 1990; Wimalawansa 1996).
CGRP has been studied to determine its impacts on appetite and feeding behavior. CGRP injected intracerebroventricularly (ICV) was observed to reduce food intake for a 24-h period in a rat (Krahn et al. 1984) as well as in an avian model (Cline et al. 2009).
Based on CGRP-mediated central anorexigenic effects and because CGRP and its receptors are localized in the gastric mucosa, we hypothesized that CGRP administered peripherally could regulate feeding behavior, energy balance, and metabolism. Hence, the aim of this study was to investigate the effects of a single intraperitoneal (IP) treatment with CGRP on appetite/satiety, feeding and drinking behavior, respirometry, energy expenditure, and physical activity in wild-type (WT) mice. In addition, we evaluated the impact of an IP treatment with CGRP on plasma metabolic hormone levels to elucidate the potential mechanism underlying CGRP’s effects on body energy homeostasis and metabolism.
Methods
Animals and Diets
To investigate the effects of an IP dose of CGRP on appetite, feeding behavior, and metabolism, C57/Bl6 WT, sex-and age-matched mice (n = 32) were studied. Mice were single housed under controlled light cycle illumination (0600–1800) and temperature (21–23 °C) conditions, fed a standard diet (Prolab RMH 2500; LabDiet, Saint Louis, MO), and given ad libitum access to water dispenser and food hopper throughout the study. The animal protocol of this experimental study was approved by the Institutional Animal Care and Use Committee (IACUC) of the VA Greater Los Angeles Healthcare System.
Experimental Design
WT mice (10–12 weeks of age) were divided into two experimental groups: one group was treated with CGRP and the other with saline. Each mouse, housed individually in a Promethion home-base cage, was injected at the beginning of the dark cycle (1800) with 2 nmol of CGRP in 200 μl of saline (n =16) or with 200 μl of saline as control (n = 16). Directly following the injection, data collection began. Food and water consumption, physical activity, and calorimetry parameters were measured continuously for 48 h following the treatment. At the beginning of the study, a CGRP dose response assay was used to select the 2 nmol dose on the basis of the significant effects observed on food intake, respirometry parameters, and total energy expenditure, whereas a 0.2 nmol dose of CGRP, as well as the vehicle injection, did not produce significant changes in the studied parameters.
Determination of Food Intake Behavior
Analyses of food and water intake were performed using an automated Promethion metabolic system (Sable Systems, Las Vegas, NV), allowing for continuous, undisturbed, real-time monitoring of the food hopper and water bottle. After injection, complete food/water consumption and feeding/drinking behavior were characterized. Cumulative food/water intake, bout/meal frequency, total time spent eating and drinking (time in minutes or percentages), bout/meal size, duration, and eating rate were measured. “Mean food intake” is defined as the total food intake measured during a period divided by the number of bouts. Food intake was measured undisturbed in real time through a weight sensor with a 3-mg resolution that was attached to a food hopper. Total water intake was calculated as total grams of water consumed during either the dark or light phase. Bouts are defined as the number of times the mouse drank water or consumed food. Mean water intake was calculated as total water intake divided by the number of bouts and minutes that each animal spent drinking.
Assessment of Indirect Calorimetry Using the Promethion Metabolic System
Indirect calorimetry data were recorded in the experimental animals using a Promethion metabolic cage system (Sable Systems, Las Vegas, NV) as described previously (Vu et al. 2017). Mice were single-housed and acclimated for 5 days in Promethion metabolic cages. Then, mice were IP injected with either 2 nmol of CGRP in 200 μl of saline (n = 16), or 200 μl of saline (n = 16) as control, and calorimetric parameters were recorded. Respiratory gases and water vapor were measured with an integrated fuel cell oxygen analyzer, spectrophotometric CO2 analyzer, and a capacitive water vapor partial pressure analyzer (Lighton 2008). Respiratory quotient (RQ) was calculated as the ratio of CO2 production over O2 consumption. Energy expenditure was calculated using the Weir equation: kcal/h = 60 × (0.003941 × VO2 + 0.001106 × VCO2) (Weir 1949).
Plasma Blood Samples
Each group of mice (n =16 each), fasted overnight, was IP injected with either CGRP (2 nmol in 200 μl of saline) or saline (200 μl as control. Whole blood samples were collected 30 min post-injection. Plasma samples, obtained from the whole blood collection, were added with a cocktail of protease inhibitors including inhibitor cocktail tablets with EDTA (Roche, Indianapolis, IN), aprotinin (Pittsburgh, PA), and dipeptidyl peptidase IV (DPP-IV) inhibitor (Millipore, Billerica, MA).
Measurement of Plasma Hormone Levels
Plasma levels of a selected panel of metabolic hormones were measured using a mouse metabolic hormone magnetic assay kit (Millipore) according to the manufacturer’s instructions. The hormones included in the panel were active ghrelin, glucagon-like peptide-1 (GLP-1), glucagon, insulin, leptin, peptide YY (PYY), and resistin. Each sample was assayed in duplicate on a 96-well plate. Analysis of quality control standards provided in the kit matched expectations, and the assay had an interassay precision of < 25% and an intraassay precision of < 7%.
Data Analysis and Trial Exclusion
A two-way analysis of variance (ANOVA) was used to evaluate the statistical significance of RQ, VO2, VCO2, and kcal between the two experimental treatment groups. Factors included in the two-way ANOVA were treatment (CGRP, vehicle), categorical time (30-min intervals), and an interaction term (food intake by time). The area under the curve (AUC) for RQ, VO2, VCO2, and kcal values was calculated, and t test was used to determine the statistical significance between the two treatment groups. Food and water consumption were analyzed by a multiple t test. Metabolic hormone levels were analyzed using an unpaired multiple t test. Graphs were constructed and standard error of the mean values plotted using GraphPad Prism 6/7 Software (La Jolla, CA).
Results
CGRP Peripheral Treatment Alters Food and Water Consumption in Mice
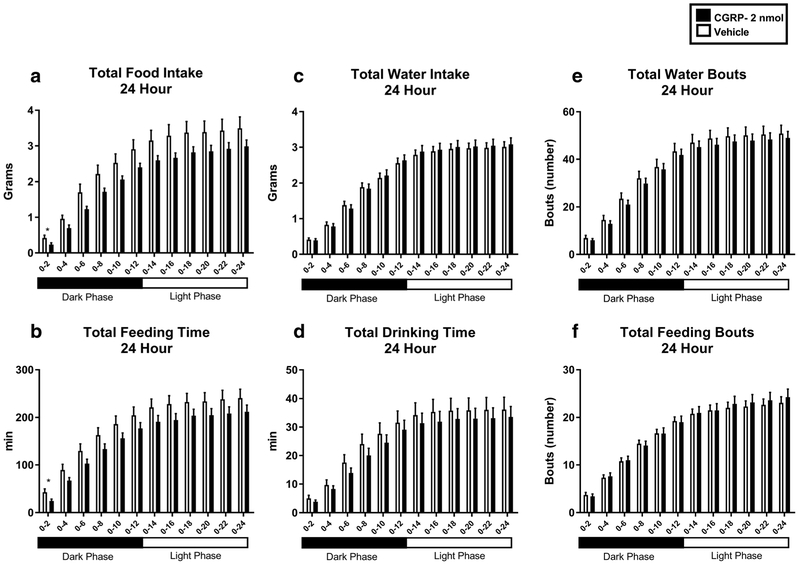
CGRP IP injected in WT mice at a dose of 2 nmol in 200 μl of saline significantly reduced cumulative food intake (Fig. 1). In CGRP-treated mice, an analysis of the 24-h period posttreatment revealed a significant decrease of food consumption from time 0 to 2 h post-injection (0.2338 g vs. 0.4214 g, p < 0.05) (Fig. 1a). Additionally, an analysis conducted by subdividing the 24-h post-treatment period in 2-h time intervals revealed that CGRP treatment reduced the cumulative time that mice spent eating during the interval from 0 to 2 h post-injection as compared to saline injected controls (24.084 min vs. 42.6521 min, p < 0.05) (Fig. 1b). Water intake, expressed in cumulative grams over 24 h post-treatment (Fig. 1c) as well as cumulative time spent drinking, expressed in minutes (Fig. 1d), showed no significant differences between the two treatment groups. No significant differences in the number of drinking bouts (Fig. 1e) and feeding bouts (Fig. 1f) were found between the CGRP and vehicle-injected animals in the 24-h post-treatment period, subdivided in 2-h time intervals.
Fig. 1.
Water intake and feeding behavior in WT mice IP injected with CGRP 2 nmol (n = 16) (black) or saline (n = 16) (white). CGRP significantly reduced cumulative food intake (a) at 0–2 h post-injection and cumulative time spent eating (b) at 0–2 h post-injection. Cumulative water intake (c), cumulative time spent drinking (d), and total water bouts (e) were not significantly altered by CGRP treatment as well as total feeding bouts (f). Data are expressed as mean ± SEM. *p < 0.05
CGRP Peripherally Regulates RQ, VCO2, and VO2 in a Murine Model
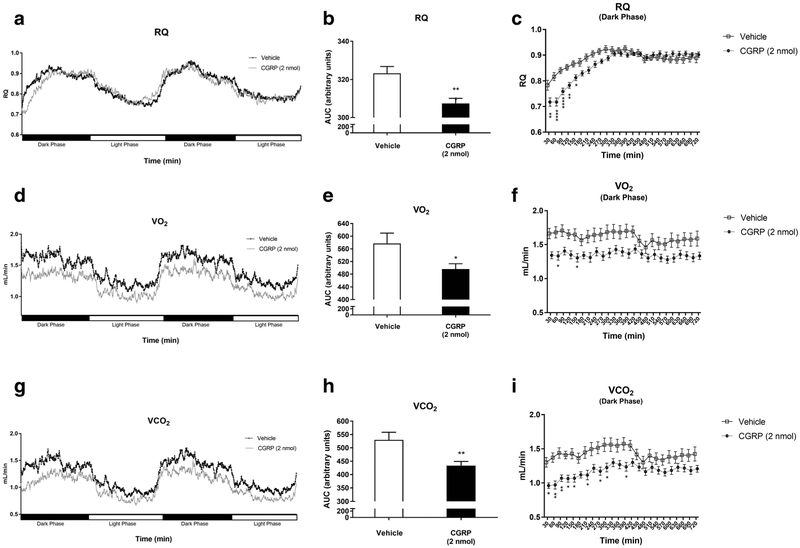
An indirect calorimetry study showed that CGRP-injected mice had significantly altered parameters as compared to vehicle-injected mice. Cumulatively, during the first 720 min after treatment (dark cycle), CGRP-injected mice, as compared to vehicle-treated mice, had a significantly lower respiratory quotient (RQ) [307.1 ± 12.10 vs. 323.3 ± 15.11 (p < 0.01)] as shown in Fig. 2a, b. In addition, there was a lower VO2 (490.5 ± 70.64 vs. 577.0 ± 138.4, p < 0.05) as shown in Fig. 2d, e. Furthermore, these mice also showed a lower VCO2 (432.0 ± 68.16 vs. 529.8 ± 118.4, p < 0.01) as shown in Fig. 2g, h. An analysis of the 24-h post-injection time period, subdivided into 30-min intervals, indicated that CGRP-treated mice had significantly lower RQ at 30 (0.7176 vs. 0.7831, p < 0.01), at 60 (0.7178 vs. 0.8167, p < 0.0001), at 90 (0.7590 vs. 0.8406,p < 0.0001), at 120 (0.7829 vs. 0.8528, p < 0.01), and at 150 min (0.8127 vs. 0.8664, p < 0.05) as compared to vehicle-injected mice (Fig. 2c). Additionally, CGRP-treated mice had significantly lower VO2 at 60 (1.334 vs. 1.681, p < 0.05) and at 120 min (1.349 vs. 1.652, p < 0.05) compared to vehicle-injected mice (Fig. 2f). CGRP-injected mice also had a significantly lower VCO2 at 30 (0.9608 vs. 1.302, p < 0.05), at 60 (0.9703 vs. 1.371, p < 0.01), at 90 (1.069 vs. 1.438, p < 0.01), at 120 (1.063 vs. 1.408, p < 0.05), at 150 (1.069 vs. 1.428, p < 0.05), at 210 (1.116 vs. 1.446, p < 0.05), at 270 (1.172 vs. 1.515, p< 0.05), at 300 (1.226 vs. 1.558, p< 0.05), and at 390 min (1.236 vs. 1.573, p < 0.05) compared to vehicle-injected mice (Fig. 2i). No significant differences in RQ, VCO2, and VO2 were found between males and females injected with CGRP.
Fig. 2.
Analysis of respirometry and calorimetry data in WT mice IP injected with CGRP 2 nmol (n = 16) (black) or saline (n = 16) (white). A 48-h analysis of RQ (a) was conducted by calculating the total area under the curve for the dark phase (b) that was also subdivided in 30-min intervals (c) in CGRP and vehicle-injected mice. A 48-h analysis of VO2 (d) was conducted by calculating the total area under the curve for the dark phase (e) that was also subdivided in 30-min intervals (f) in CGRP and vehicle-injected mice. A 48-h analysis of VCO2 (g) was conducted by calculating the total area under the curve for the dark phase (h) that was also subdivided in 30-min intervals (i) in CGRP and vehicle-injected mice. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
CGRP Does Not Alter Physical Activity in Mice
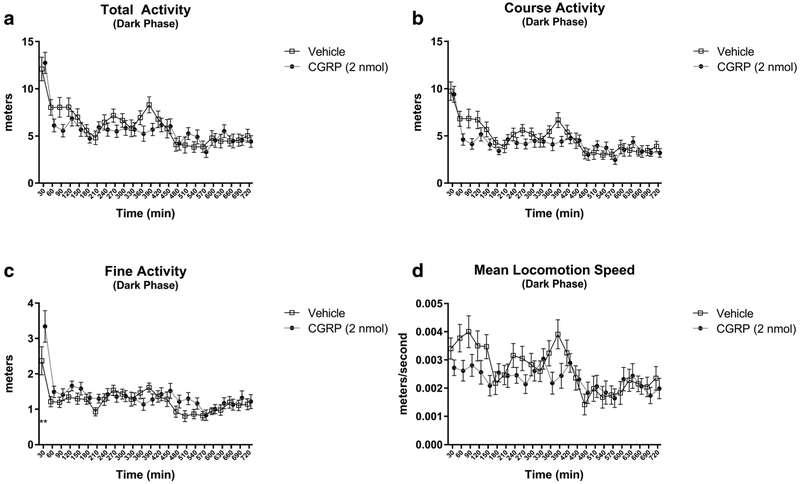
To determine whether the effects of CGRP were mediated by altered physical activity levels, we measured physical activity in the study mice by utilizing the Promethion laser-guided physical activity monitor. Physical activity was analyzed in each experimental group by subdividing the 24-h time post-injection into 30-min intervals (Fig. 3). No significant differences in total activity (Fig. 3a), course activity (Fig. 3b), and mean locomotion speed (Fig. 3d) were found in CGRP-injected mice as compared to vehicle-injected controls. CGRP-injected mice had a significant increase in their fine activity as compared to vehicle during the first 30 min post-injection (3.343 m vs. 2.370 m, p < 0.01), as shown in Fig. 3c. All other time interval values were not significant.
Fig. 3.
Analysis of physical activity in WT mice IP injected with CGRP 2 nmol (n = 16) (closed circle) or saline (n = 16) (open square) IP-injected WT mice. CGRP treatment did not significantly alter total activity (a), course activity (b) or mean locomotion speed (c), while significantly increasing fine activity (d) as compared to saline control. Data are expressed as mean ± SEM. **p < 0.01
CGRP Peripherally Regulates Energy Expenditure in Mice
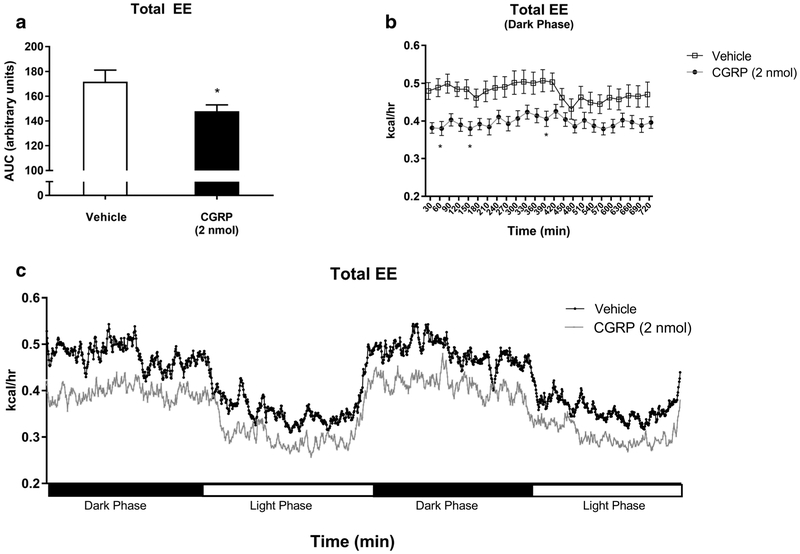
CGRP IP treatment significantly altered energy expenditure compared to vehicle, as shown in Fig. 4. Cumulatively, during the first 720 min after treatment (dark cycle), CGRP-injected mice had significantly lower total energy expenditure (TEE) compared to vehicle-injected controls, as shown in Fig. 4a, c (146.4 kcal/h vs. 171.7 kcal/h, p < 0.05). A study performed by subdividing the 24-h post-injection time period into 30-min intervals showed that CGRP-injected mice, compared to vehicle-injected controls, had a significant decrease in TEE at 60 (0.3814 kcal/h vs. 0.4796 kcal/h, p < 0.05), at 150 (0.3796 kcal/h vs. 0.4843 kcal/h, p < 0.05), and at 390 min (0.4055 kcal/h vs. 0.5068 kcal/h, p < 0.05) as shown in Fig. 4b.
Fig. 4.
An analysis of total energy expenditure (TEE) was conducted in WT mice IP injected with CGRP 2 nmol (n =16) (black) or saline (n = 16) (white) by calculating for the dark phase: the total area under the curve (a), 30-min time intervals (b), and 48-h TEE (c). Data showed a significant reduction in TEE in CGRP-treated mice as compared to control. Data are expressed as mean ± SEM. *p < 0.05
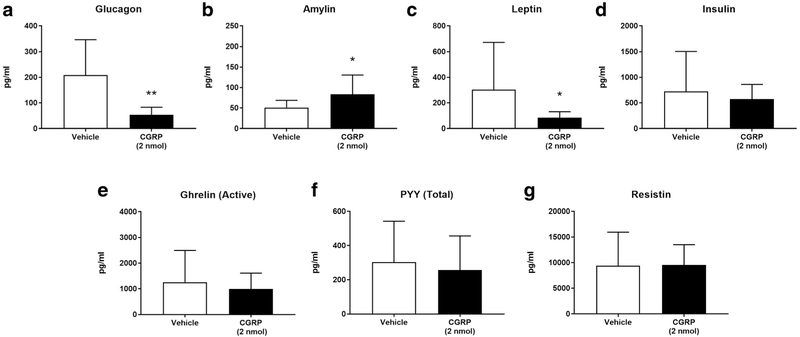
CGRP Administration Suppresses Glucagon and Leptin
Plasma hormone levels were assayed in the two murine experimental groups. CGRP-injected mice, as compared to saline-injected mice, had significantly lower plasma levels of glucagon when comparing combined female and male groups (209.0 pg/ml vs. 52.93 pg/ml, p < 0.01) (Fig. 5a) as well as separated male only (230.6 pg/ml vs. 36.63 pg/ml, p < 0.05) (Table 1) or female only groups (187.4 pg/ml vs. 66.51 pg/ml, p < 0.05) (Table 1). Plasma levels of amylin were significantly increased in CGRP-treated mice when comparing combined female and male groups (50.87 pg/ml vs. 83.69 pg/ml, p < 0.05) (Fig. 5b) as well as only female groups (43.59 pg/ml vs. 79.83 pg/ml, p < 0.05) (Table 1), but not male groups (Table 1). Plasma leptin levels were significantly lower in CGRP-injected female and male combined (303.6 pg/ml vs. 84.58 pg/ml, p < 0.05) (Fig. 5c) as well as in only female groups (170.8 pg/ml vs. 78.94 pg/ml, p < 0.05) (Table 1) but not in male groups (Table 1). Plasma levels of insulin (Fig. 5d), ghrelin (active) (Fig. 5e), PYY (total) (Fig. 5f), and resistin (Fig. 5g) showed no significant differences in CGRP-injected male or female groups (Table 1).
Fig. 5.
An analysis of a panel of metabolic hormones was conducted in fasted WT male and female mice IP injected with CGRP 2 nmol [n = 16 (8m, 8f)] (black) or saline as control [n = 16 (8m, 8f)] (white). Plasma levels (pg/ml) of glucagon (a), amylin (b), leptin (c), insulin (d), ghrelin (active) (e), PYY (total) (f), and resistin (g) in combined male and female group are shown in Fig. 5
Table 1.
CGRP (2 nmol) administration alters plasmatic hormones
| Female (pg/ml) |
Male (pg/ml) |
Combined (pg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CGRP | Vehicle | p | CGRP | Vehicle | p | CGRP | Vehicle | p | |
| Glucagon | 66.51 ±14.56 | 187.4 ±44.71 | * | 36.63 ±2.89 | 230.6 ±53.81 | * | 52.93 ±9.027 | 209.0 ±34.24 | ** |
| Amylin | 79.83 ±16.44 | 43.59±2.91 | * | 87.54 ±17.83 | 58.14 ±7.835 | ns | 83.69 ±11.76 | 50.87 ±4.452 | * |
| Leptin | 78.94 ±14.92 | 170.8 ±38.95 | * | 194.7 ±104.3 | 364.0 ±183.9 | ns | 84.58 ±12.38 | 303.6 ±94.88 | * |
| Insulin | 518.1 ±120.1 | 929.9 ±443.4 | ns | 629.3 ±84.4 | 550.1 ±151 | ns | 573.7 ±72.35 | 725.4±216.7 | ns |
| Ghrelin (active) | 1264±238.5 | 1246 ±480.6 | ns | 724.1 ±157.6 | 1172 ±419.4 | ns | 993.9±154.7 | 1259 ±308.9 | ns |
| PYY (total) | 189.5 ±35.77 | 381.1 ±101.9 | ns | 323.9 ±90.34 | 225.0 ±57.11 | ns | 256.7 ±50.04 | 303.0±59.92 | ns |
| Resistin | 11,203 ±1287 | 8616 ± 2715 | ns | 8094 ±1377 | 10,153 ±2008 | ns | 9559 ±986 | 9385 ±1643 | ns |
In Table 1, plasma levels (pg/ml) of glucagon, amylin, leptin, insulin, ghrelin (active), PYY (total), and resistin in separated male or female groups are shown. Data are expressed as mean ± SEM
p < 0.05,
p < 0.01
Discussion
This study investigates the effects of an IP treatment with CGRP on feeding and drinking behavior, respiratory gases, energy expenditure, physical activity, and plasma metabolic hormones. The data show that a single dose of CGRP significantly decreased food consumption and altered calorimetric parameters and plasma metabolic hormones but not physical activity, thus confirming that CGRP plays a pivotal role in the regulation of metabolism.
CGRP was previously shown to regulate body metabolism centrally through glucose homeostasis (Rossetti et al. 1993), insulin sensitivity (Leighton and Cooper 1988), and appetite (Carter et al. 2013). Previous studies reported a reduction of food intake following ICV injection of CGRP in a rat (Krahn et al. 1984; Lutz et al. 1998) as well as in an avian model (Cline et al. 2009). Similarly, CGRP administration in the intraparaventricular nucleus reduced food intake in fasted male rats, an effect that was attenuated by the antagonist CGRP8–37 (Dhillo et al. 2003). Furthermore, activation of parabrachial neurons expressing CGRP in mice caused anorexia, an effect that was ameliorated by AgRP neuron ablation (Carter et al. 2013). Studies of alpha-CGRP−/− mice, fed by standard diet, showed increased food intake and higher core temperatures and energy expenditure, but lower RQ, thus confirming that CGRP is an important modulator of metabolism (Liu et al. 2017; Walker et al. 2010). In the current study, a single IP dose of CGRP (2 nmol) significantly reduced food intake and total meal duration for up to 2 h after treatment. These data agree with the increased food consumption observed in CGRP genetic null models and the decreased food intake by central or peripheral injection (Morley et al. 1996; Sun et al. 2010; Walker et al. 2010).
Oral, intravenous, or intracisternal administration of CGRP in dogs (Pappas et al. 1986), rats (Raybould et al. 1988), rabbits (Taché et al. 1991), and cods (Shahbazi et al. 1998) inhibited gut motility and gastric acid secretion; thus, it was suggested that the CGRP-induced reduction in food intake could be due to a delayed gastric emptying (Martinez and Taché 2006). However, given that food and water consumption are directly correlated (Bachmanow et al. 2002), lower food consumption is usually associated with decreased water consumption. Our study found that CGRP-induced decrease of food intake was not linked to decreased water intake; therefore, we postulate that CGRP’s anorexia is due to other metabolic effects. Previous research in animal models showed that CGRP reduced food but not water intake in rat and mouse models (Krahn et al. 1984; Morley et al. 1996; Sun et al. 2010), thus supporting our findings that the CGRP-induced decrease of food and water intake is not associated.
Previous research evaluated CGRP’s impact on energy expenditure and physical activity. In our study, a significant decrease in TEE was measured during the dark/active cycle in CGRP-injected mice when analyzing 30-min intervals post-injection. Our findings are supported by other authors that reported higher TEE values in maleαCGRP knockout mice fed either a normal or high fat diet (Liu et al. 2017; Walker et al. 2010). In our study, no significant differences were observed between the two treatment groups in total activity, coarse activity, or mean locomotion speed, thereby indicating that CGRP decreased energy expenditure without impacting physical activity.
Since CGRP is one of the most potent vasodilators (Brain et al. 1985), causing hypotension in mice (Russell et al. 2014), we speculated that CGRP-induced vasodilation could be correlated to the lower energy expenditure. Previously, CGRP-induced vasodilation and hypotension were considered a limitation for its potential clinical use (Hüttemeier et al. 1993); however, our data indicate that CGRP-treated mice retained physical activity levels equivalent to the controls and had only a limited, though significant, decrease in TEE.
Our study also assessed the impact of IP-injected CGRP on VO2, VCO2, and RQ. Previous studies reported increased VO2 and VCO2 in CGRP−/− mice (Liu et al. 2017; Walker et al. 2010), which is consistent with the decreased VO2 and VCO2 observed in our CGRP-treated animals. We also found that CGRP significantly reduced RQ during nighttime (active phase). Other authors found in CGRP−/− mice daytime (non-active) reduced RQ (Walker et al. 2010); however, in genetic CGRP knockout models, compensatory pathways likely develop and alter metabolic pathways; therefore, it is difficult to directly compare peptide genetic knockouts with injected murine models.
The RQ values measured in our CGRP-injected mice indicate enhanced lipid metabolism, thereby suggesting that CGRP promotes the use of stored lipids as energy source in response to lower caloric intake. Data from Danaher et al. (2008) demonstrated that αCGRP systemically stimulated lipid beta-oxidation, thus supporting our findings. These combined data suggest that CGRP regulates energy intake/balance and metabolism through mechanisms other than a reduced gastric function, as previously published (Martinez and Taché 2006).
We postulate that the CGRP-induced lower appetite and intake of food including carbohydrates can account for the decreased VCO2, VO2, and RQ. CGRP-treated mice maintained levels of activity similar to the controls and consumed oxygen to maintain oxidative phosphorylation and body homeostasis. However, the CGRP-induced reduction of food and carbohydrate intake could explain the more significant reduction in VCO2 compared to VO2.
Additionally, no “rebound effect,” consisting of a later reversion of the initial effect, was measured in CGRP-injected mice. For example, the RQ values, significantly altered upon CGRP injection, returned to basal after 6 h.
An analysis of plasma metabolic hormone levels revealed lower glucagon in overnight-fasted, CGRP-treated males and females (Fig. 5a and Table 1). Conversely, Sun et al. (2010) reported increased plasma glucagon in 24-h fasted rats by IP CGRP, thus suggesting that CGRP induce opposite effects in different rodent models. In a rat model, Pettersson and Ahrén (1988) found increased basal glucagon but inhibited glucose-stimulated glucagon upon IV infusion of CGRP, whereas Yamaguchi et al. (1990), using a CGRP IV treatment in conscious male rats, reported no changes in glucagon. In addition, an in vitro study, using dog intestine perfused with CGRP, showed no significant changes in glucagon secretion (Hermansen and Ahrén 1990). Overall, these controversial data on CGRP’s effect on plasma glucagon could be attributed to the different animal models and blood collection pro-cedures (Kraenzlin et al. 1985; Pettersson and Ahren 1988; Sun et al. 2010).
In our study, plasma insulin levels were not modified by CGRP in both females and males (Fig. 5d, Table 1). Conversely, Sun et al. (2010) described that CGRP IP injected in 24-h fasted rats decreased insulin. In an in vitro study, using a perfused dog intestine model, insulin secretion was inhibited at lower CGRP concentrations, but stimulated at higher (Hermansen and Ahrén 1990). Furthermore, Pettersson and Ahrén (1988) reported increased basal insulin secretion and inhibition of glucose-stimulated insulin secretion after IV infusion of CGRP in rats. Conversely, Yamaguchi et al. (1990) described that IV-administered CGRP dose-dependently increased plasma insulin in rats. In another study, patients fasted overnight and then IV infused with rat CGRP had normal insulin (Kraenzlin et al. 1985). Overall, these data suggest that CGRP modulates insulin secretion in rats, but not in mice or humans.
Our data showed lower leptin levels in CGRP-injected females, but not males (Fig. 5c, Table 1), thereby suggesting gender-specific differences in secretion. Another study in 24-h fasted rats, IP injected with CGRP, reported unchanged plasma leptin levels; however, only male rats were included (Sun et al. 2010). In leptin knockout ob/ob mice, a éCGRP analog long-term treatment did not affect either body weight or composition (Nilsson et al. 2016), thus indicating that the leptin pathway mediates CGRP’s effect on metabolism.
We measured elevated plasma levels of amylin only in CGRP-treated females but not in males (Table 1). CGRP, as well as amylin, regulate insulin secretion and glycogenesis and impair glucose uptake and insulin functions in vitro (Kreutter et al. 1993; Tanaka et al. 2013). Our data suggest a potential effect of sex hormones on the CGRP pathway leading to amylin secretion, but further studies are necessary to elucidate these mechanisms. Active-ghrelin levels were not significantly changed in CGRP-injected mice (Fig. 5e, Table 1). An in vitro study by Nilsson et al. (2016) reported that five Gαs-coupled receptors are involved in ghrelin secretion, including the main CGRP receptor, a heterodimer of RAMP1 and CLR, and that increasing CGRP concentrations correspond with higher ghrelin secretion.
In our experimental animals, PYY levels were not significantly altered by CGRP (Fig. 5f). No other in vivo studies have examined CGRP effects on PYY secretion; however, an in vitro study reported higher PYY secretion in rat colon perfused with CGRP (Plaisancié et al. 2009). Similarly, plasma resistin levels demonstrated no significant differences in overnight-fasted, CGRP-treated males or females (Fig. 5g); however, Liu et al. (2017) found higher plasma resistin in αCGRP knockout mice.
We considered the possibility that peripherally administered CGRP could cross the blood-brain barrier (BBB) and act centrally. Other studies have determined that abluminally administered CGRP crossed the BBB; however, luminally administered CGRP was prevented from reaching arterial smooth muscle cells in the brain (Edvinsson et al. 2007; Edvinsson and Tfelt-Hansen 2008). In our current study, we cannot rule out that IP-injected CGRP crossed the BBB and acted centrally.
Previous research has established that physiological CGRP plasma concentrations in human subjects are 10–15 pmol/l (Juhasz et al. 2003; Kraenzlin et al. 1985, see below). Given that the dose of 2 nmol used in our experiments is much higher than the circulating CGRP concentrations, we propose that the significant effects observed on a number of metabolic parameters in our study are pharmacological and not physiological.
In conclusion, our study supports an anorexigenic role for CGRP in the regulation of the digestion and absorption of nutrients, metabolism, and body homeostasis. The lack of a rebound effect after CGRP administration would support a potential use of this peptide to reduce appetite and promote the beta oxidation of lipids as energy source in subjects with overeating and obesity disorders. Future studies are necessary to analyze the effects of a long-term treatment with CGRP in animal models of hyperphagia and obesity.
Acknowledgments
Grants VA Merit Review I01RX000873 (Germano, P).
VA ShEEP IS1BX003075 (Germano, P).
VA ShEEP IS1BX003553 (Germano, P).
NIH NIDDK P30 NIH DK41301.
NIH NIDDK U01 CSCPDPC -CCs -RFA-DK-14-027.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adult Obesity Facts Centers for Disease Control and Prevention (2018) https://www.cdc.gov/obesity/data/adult.html
- Bachmanow AA, Reed DR, Beauchamp GK, Tordoff MG (2002) Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature 313: 3–9 [DOI] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD (2013) Genetic identification of a neural circuit that suppresses appetite. Nature 503:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MA, Calchary WA, Nandar W (2009) Effect of calcitonin gene-related peptide (CGRP) on avian appetite-related processes. Behav Brain Res 196:242–247 [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sernini C, Bunnett NW, Grady EF (2005) Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 490:239–255 [DOI] [PubMed] [Google Scholar]
- Danaher RN, Loomes KM, Leonard BL, Whiting L, Hay DL, Xu LY, Kraegen EW, Phillips AR, Cooper GJ (2008) Evidence that alpha-calcitonin gene-related peptide is a neurohormone that controls systemic lipid availability and utilization. Endocrinology 149:154–160 [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Small CJ, Jethwa PH, Russell SH, Gardiner JV, Bewick GA, Seth A, Murphy KG, Ghatei MA, Bloom SR (2003) Paraventricular nucleus administration of calcitonin gene-related peptide inhibits food intake and stimulates the hypothalamo-pituitary-adrenal axis. Endocrinology 144:1420–1425 [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Tfelt-Hansen P (2008) The blood-brain barrier in migraine treatment. Cephalalgia: An International Journal of Headache 28: 1245–1258 [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Nilsson E, Jansen-Olsen I (2007) Inhibitory effect of BIBN4096BS, CGRP8–37, a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol 150:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood HC, Bloom SR, Murphy KG (2011) Peptides and their potential role in the treatment of diabetes and obesity. Society for Biomedical Diabetes Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MB (2003) Neurohumoral control of gastrointestinal motility. Physiol Res 52:1–30 [PubMed] [Google Scholar]
- Hermansen K, Ahrén B (1990) Dual effects of calcitonin gene-related peptide on insulin secretion in the perfused dog pancreas. Regul Pept 27:149–157 [DOI] [PubMed] [Google Scholar]
- Hüttemeier PC, Ritter EF, Benveniste H (1993) Calcitonin gene-related peptide mediates hypotension and tachycardia in endotoxic rats. Am J Phys 265:767–769 [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Modos EA, Olajos S, Jakab B, Nemeth J, Szolcsanyi J, Vitrai J, Bagdy G (2003) NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. International Association for the Study of. Pain 106:461–470 [DOI] [PubMed] [Google Scholar]
- Kaiser EA, Russo AF (2013) CGRP and migraine: could PACAP play a role too? Neuropeptides 47:451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K, Ishihara S, Karim Rumi MA, Moriyama N, Kazumori H, Suetsugu H, Sato H, Fukuda R, Adachi K, Shibata M, Onodera S, Chiba T, Kinoshita Y (2002) Localization of calcitonin gene-related peptide receptors in rat gastric mucosa. Peptides 23:955–966 [DOI] [PubMed] [Google Scholar]
- Kraenzlin ME, Ch’ng JLC, Mulderry PK, Ghatei MA, Bloom SR (1985) Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul Pept 10:189–197 [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Levine AS, Morley JE (1984) Effects of calcitonin gene-related peptide on food intake. Peptides 5:861–864 [DOI] [PubMed] [Google Scholar]
- Kreutter DK, Orena SJ, Torchia AJ, Contillo LG, Andrews GC, Stevenson RW (1993) Amylin and CGRP induce insulin resistance via a receptor distinct from cAMP-coupled CGRP receptor. Am J Phys 264:606–613 [DOI] [PubMed] [Google Scholar]
- Leighton B, Cooper GJ (1988) Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature 335:632–635 [DOI] [PubMed] [Google Scholar]
- Lennerz JK, Rühle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K (2008) Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 507:1277–1299 [DOI] [PubMed] [Google Scholar]
- Lighton JRB (2008) Measuring metabolic rates: a manual for scientists, 1st edn. Oxford University Press, New York [Google Scholar]
- Liu T, Kamiyoshi A, Sakurai T, Ichikawa-Shindo Y, Kawate H, Yang L, Tanaka M, Xian X, Imai A, Zhai L, Hirabayashi K, Dai K, Tanimura K, Liu T, Cui N, Igarashi K, Yamauchi A, Shindo T (2017) Endogenous calcitonin gene-related peptide regulates lipid metabolism and energy homeostasis in male mice. Endocrinology 158: 1194–1206 [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E (1998) Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides 19:1533–1540 [DOI] [PubMed] [Google Scholar]
- Martinez V, Taché Y (2006) Calcitonin gene-related peptide and gastrointestinal function Handbook of Biologically Active Peptides 138: 1005–1011 [Google Scholar]
- Morley JE, Farr SA, Flood JF (1996) Peripherally administered calcitonin gene-related peptide decreases food intake in mice. Peptides 17: 511–516 [DOI] [PubMed] [Google Scholar]
- Nilsson C, Hansen TK, Rosenquist C, Hartmann B, Kodra JT, Lau JF, Clausen TR, Raun K, Sams A (2016) Long acting analogue of the calcitonin gene-related peptide induces positive metabolic effects and secretion of the glucagon-like peptide-1. Eur J Pharmacol 773:24–31 [DOI] [PubMed] [Google Scholar]
- Pappas T, Debas HT, Walsh JH, Rivier J, Taché Y (1986) Calcitonin gene-related peptide-induced selective inhibition of gastric acid secretion in dogs. Am J Phys 250:127–133 [DOI] [PubMed] [Google Scholar]
- Parsons AM, Seybold VS (1997) Calcitonin gene-related peptide induces the formation of second messengers in primary cultures of neonatal rat spinal cord. Synapse 26:235–242 [DOI] [PubMed] [Google Scholar]
- Pettersson M, Ahrén B (1988) Insulin and glucagon secretion in rats: effects of calcitonin gene-related peptide. Regul Pept 23:37–50 [DOI] [PubMed] [Google Scholar]
- Plaisancié P, Bernard C, Chayvialle JA, Cuber JC (2009) Release of peptide YY by neurotransmitters and gut hormones in the isolated, Vascularly Perfused Rat Colon. Scandinavian Journal of Gastroenterology 30:568–574 [DOI] [PubMed] [Google Scholar]
- Raybould HE, Kolve E, Taché Y (1988) Central nervous system action of calcitonin gene-related peptide to inhibit gastric emptying in the conscious rat. Peptides 9:735–738 [DOI] [PubMed] [Google Scholar]
- Rossetti L, Farrace S, Choi SB, Giaccari A, Sloan L, Frontoni S, Katz MS (1993) Multiple metabolic effects of CGRP in conscious rats: role of glycogen synthase and phosphorylase. Am J Physiol 264:E1–E10 [DOI] [PubMed] [Google Scholar]
- Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94:1099–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi F, Karila P, Olsson C, Holmgren S, Conlon JM, Jensen J (1998) Primary structure, distribution, and effects on motility of CGRP in the intestine of the cod Gadus morhua. Am J Phys 275:19–28 [DOI] [PubMed] [Google Scholar]
- Sun JY, Jing MY, Wang JF, Weng XY (2010) The approach to the mechanism of calcitonin gene-related peptide-inducing inhibition of food intake. J Anim Physiol Anim Nutr 94:552–560 [DOI] [PubMed] [Google Scholar]
- Taché Y, Raybould H, Wei JY (1991) Central and peripheral actions of calcitonin gene-related peptide on gastric secretory and motor function. Adv Exp Med Biol 298:183–198 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kashiwagi R, Koizumi T (2013) Inhibition of calcitonin gene-related peptide (CGRP) has the potential to extend first-phase insulin secretion. Exp Clin Endocrinol Diabetes 121:280–285 [DOI] [PubMed] [Google Scholar]
- Vu JP, Luong L, Parsons WF, Oh S, Sanford D, Gabalski A, Lighton JRB, Pisegna JR, Germano PM (2017) Long-term intake of a high-protein diet affects body phenotype, metabolism, and Plasma Hormones in Mice. The Journal of Nutrition 147:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, Sewell MA, Ruggiero K, Phillips ARJ, Kraegen EW, Hay DL, Cooper GJS, Loomes KM (2010) Mice lacking the neuropeptide α-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 151:4257–4269 [DOI] [PubMed] [Google Scholar]
- Weir JBDV (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalawansa SJ (1990) Effects of in vivo stimulation on molecular forms of circulatory calcitonin and calcitonin gene-related peptide in man. Mol Cell Endocrinol 71:13–19 [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ (1996) Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocrinology Reviews 17:533–585 [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Porte DJ, Schwartz MW (1998) Signals that regulate food intake and energy homeostasis. Science 280: 1378–1383 [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Chiba T, Morishita T, Nakamura A, Inui T, Yamatani T, Kadowaki S, Chihara K, Fukase M, Fujita T (1990) Calcitonin gene-related peptide and induction of hyperglycemia in conscious rats in vivo. Diabetes 39:168–174 [DOI] [PubMed] [Google Scholar]
- Yarwood RE, Imlach WL, Lieu T, Veldhuis NA, Jensen DD, Klein HC, Aurelio L, Cai Z, Christie MJ, Poole DP, Porter CJH, McLean P, Hicks GA, Geppetti P, Halls ML, Canals M, Bunnett NW (2017) Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc Natl Acad Sci U S A 114: 12309–12314 [DOI] [PMC free article] [PubMed] [Google Scholar]